Nonaqueous electrolyte solution and nonaqueous electrolyte battery
A non-aqueous electrolyte and electrolyte technology, applied in the field of non-aqueous electrolyte batteries, which can solve the problems of hydrogen detachment reaction, inability to obtain sufficient characteristics, and reduction of battery voids, etc., to achieve high capacity, excellent storage characteristics and cycle characteristics, and less gas Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0187] {Preparation of non-aqueous electrolyte}
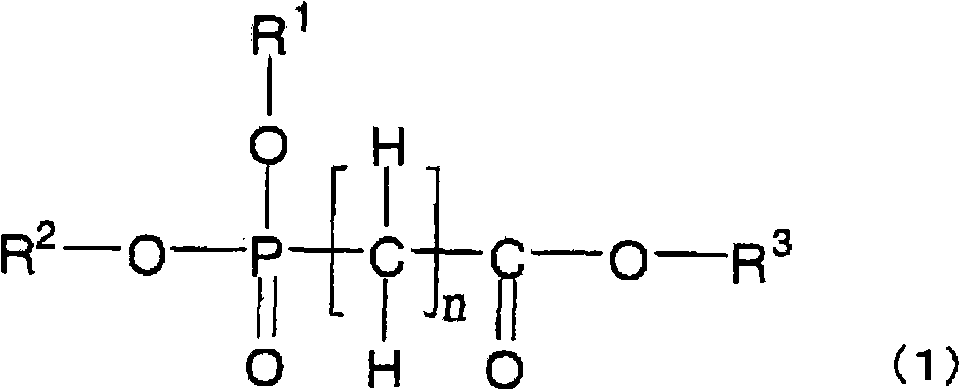
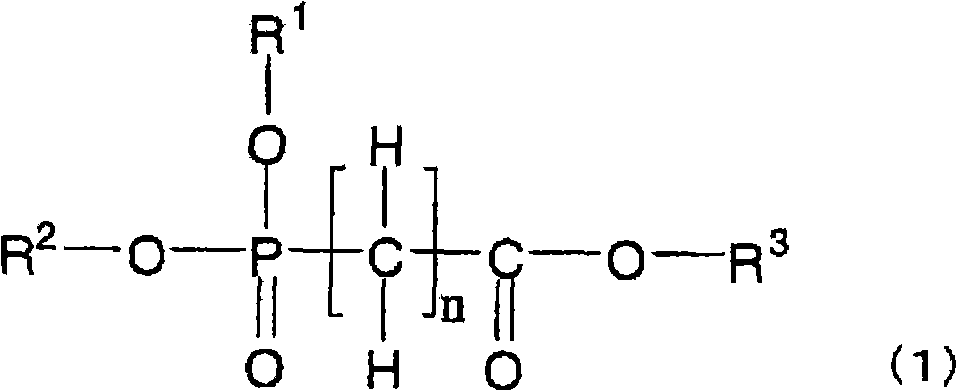
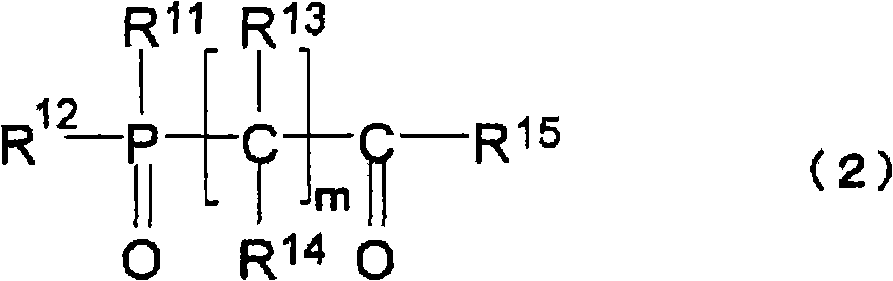
[0188] The non-aqueous electrolytic solution of the present invention can be prepared by dissolving an electrolyte, compound (1) (or compound (2) and / or compound (3)), and other compounds blended as necessary in a non-aqueous solvent. When preparing a non-aqueous electrolyte, in order to reduce the moisture content of the prepared electrolyte, each raw material is preferably dehydrated in advance, and can be dehydrated to a moisture concentration of usually below 50ppm, preferably below 30ppm, particularly preferably below 10ppm. In addition, after the electrolytic solution is prepared, dehydration, deacidification treatment, and the like may be performed.
[0189] The non-aqueous electrolytic solution of the present invention is suitable as an electrolytic solution for non-aqueous electrolytic solution batteries, especially for secondary batteries, that is, non-aqueous electrolytic solution secondary batteries (such as lithium...
Embodiment 1
[0245]
[0246] 94 parts by weight of natural graphite powder are mixed with 6 parts by weight of polyvinylidene fluoride, and N-methyl-2-pyrrolidone is added to make a slurry, and the lattice plane of the natural graphite powder in X-ray diffraction ( 002 surface) has a d value of 0.336nm, a crystallite size (Lc) of 652nm, an ash content of 0.07% by weight, a median diameter of 12μm by the laser diffraction scattering method, and a specific surface area of 7.5m by the BET method. 2 / g, the R value (=I B / I A ) is 0.12, at 1570~1620cm -1 The half width of the peak of the range is 19.9cm -1 . The slurry is evenly coated on one side of a copper foil with a thickness of 12 μm, and dried, and then, the density of the negative electrode active material layer is 1.67g / cm 3 Pressurized in a manner to make a negative electrode.
[0247]
[0248] 90 parts by weight of LiCoO 2 , 4 parts by weight of carbon black and 6 parts by weight of polyvinylidene fluoride (manufactured ...
Embodiment 2
[0254] Preparation of the electrolytic solution as in Example 1, except that triethylphosphonoacetate was used instead of triethylphosphonoformate, a sheet-shaped lithium secondary battery was made in the same manner as in Example 1, and the continuous charging characteristics and Evaluation of high temperature storage characteristics. The evaluation results are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com