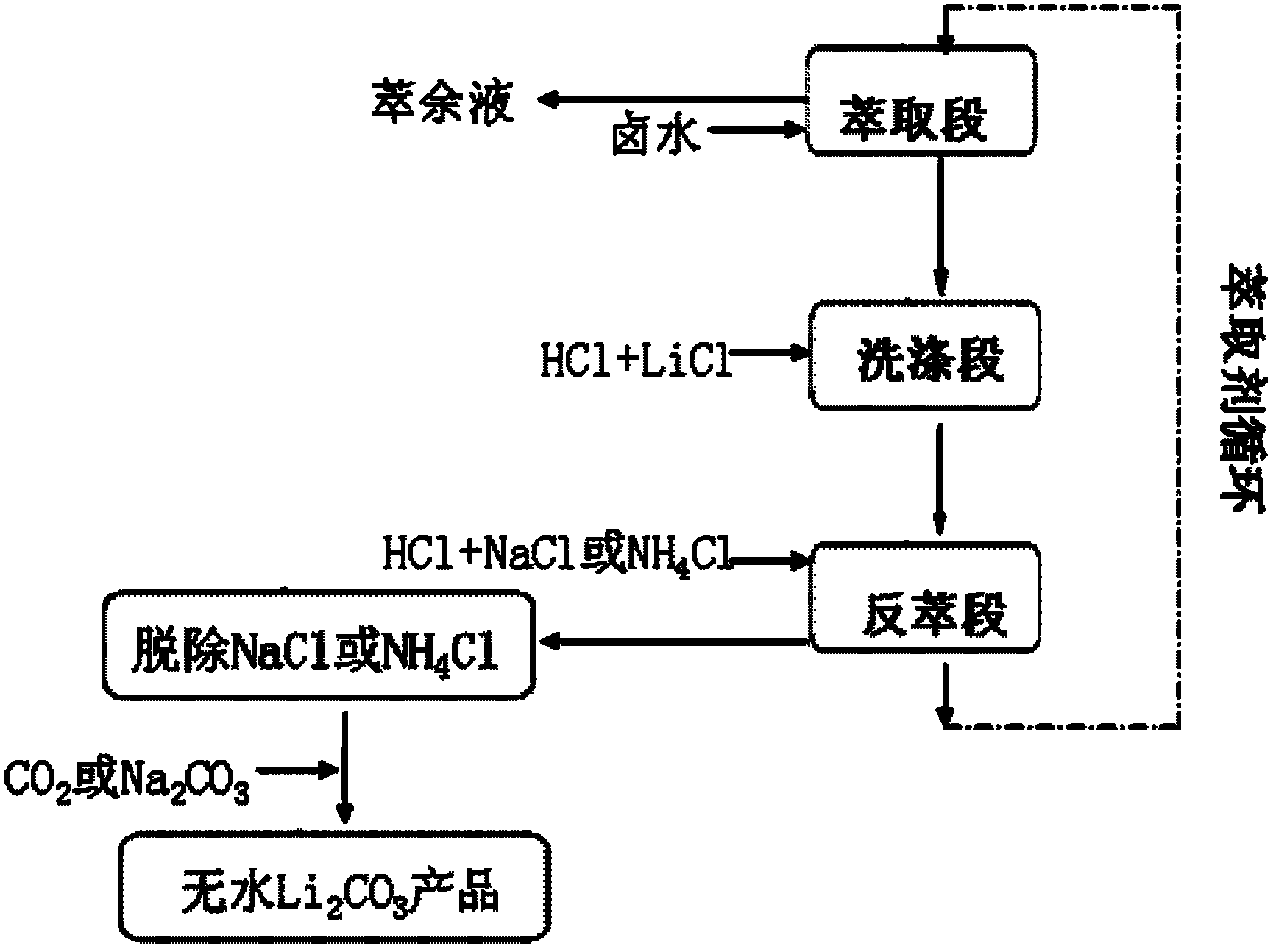
A method for extracting lithium carbonate from high magnesium-lithium ratio salt lake brine
A salt lake brine, high magnesium-lithium ratio technology, applied in lithium carbonate;/acid carbonate, nanotechnology and other directions, can solve the problems of inability to extract, easy to form third phase, low cycle process efficiency, etc. To achieve the effect of reducing acid mist and corrosion to equipment, smooth circulation process, and saving water and acid washing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The simulated salt lake brine was prepared, and the composition was lithium (0.05mol / L), magnesium (3.5mol / L) and chlorine (7.05mol / L).
[0019] The step of extracting lithium carbonate from above-mentioned simulated salt lake brine is as follows:
[0020] (1) Mix tributyl phosphate and 2,6-dimethyl-4-heptanone to make an extractant, tributyl phosphate and 2,6-dimethyl-4-heptanone respectively account for tributyl phosphate 50%, 50% of the total volume of ester and co-solvent;
[0021] (2) Iron trichloride and extractant are added successively in salt lake brine, the concentration of ferric chloride and lithium concentration ratio are 2: 1, the volume ratio of the volume of extractant and salt lake brine is 1: 1, carry out extraction ;
[0022] (3) HCl solution and LiCl solution are mixed to make detergent, and the solution concentration of HCl and LiCl is 1mol / L and 1mol / L respectively; In the organic phase that adds step (2) to separate, organic phase and detergent ...
Embodiment 2
[0027] The simulated salt lake brine was prepared, and the composition was lithium (0.05mol / L), magnesium (3.5mol / L) and chlorine (7.05mol / L).
[0028] The step of extracting lithium carbonate from above-mentioned simulated salt lake brine is as follows:
[0029] (1) Tributyl phosphate and sec-octanol are mixed to make an extractant, and tributyl phosphate and sec-octanol account for 80%, 20% of the total volume of tributyl phosphate and co-solvent respectively;
[0030] (2) Chromium trichloride and extractant are added to salt lake brine successively, the concentration of chromium trichloride and lithium concentration ratio are 2: 1, the volume ratio of the volume of extractant and salt lake brine is 2: 1, carry out extraction ;
[0031] (3) HCl solution and LiCl solution are mixed to make detergent, and the solution concentration of HCl and LiCl is 1mol / L and 1mol / L respectively; In the organic phase that adds step (2) to separate, organic phase and detergent The volume ra...
Embodiment 3
[0036] Prepare simulated salt lake brine, lithium (0.2mol / L), magnesium (4.0mol / L), sodium (0.07mol / L), potassium (0.02) and chlorine (8.29mol / L).
[0037] The step of extracting lithium carbonate from above-mentioned simulated salt lake brine is as follows:
[0038] (1) Tributyl phosphate and n-octanol are mixed to make an extractant, and tributyl phosphate and n-octanol account for 80%, 20% of the total volume of tributyl phosphate and cosolvent respectively;
[0039] (2) Iron trichloride and extractant are added to salt lake brine successively, the concentration of ferric chloride and lithium concentration ratio are 1.5: 1, the volume ratio of the volume of extractant and salt lake brine is 1: 1, carry out extraction ;
[0040] (3) HCl solution and LiCl solution are mixed to make detergent, and the solution concentration of HCl and LiCl is 1mol / L and 1mol / L respectively; In the organic phase that adds step (2) to separate, organic phase and detergent The volume ratio is 30:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com