Granular lithium ion sieve
A lithium ion, granular technology, applied in the field of granular lithium ion sieves, can solve the problems of poor adsorption performance of granular lithium ion sieves, difficult solid-liquid separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Equipped with reflux condenser, stirring device, N 2 Add 200ml cyclohexane (oil phase) and 0.50g starch (dispersant) in the four-necked flask of conduit, 35 ℃, N 2 Pre-mixed evenly under the atmosphere;
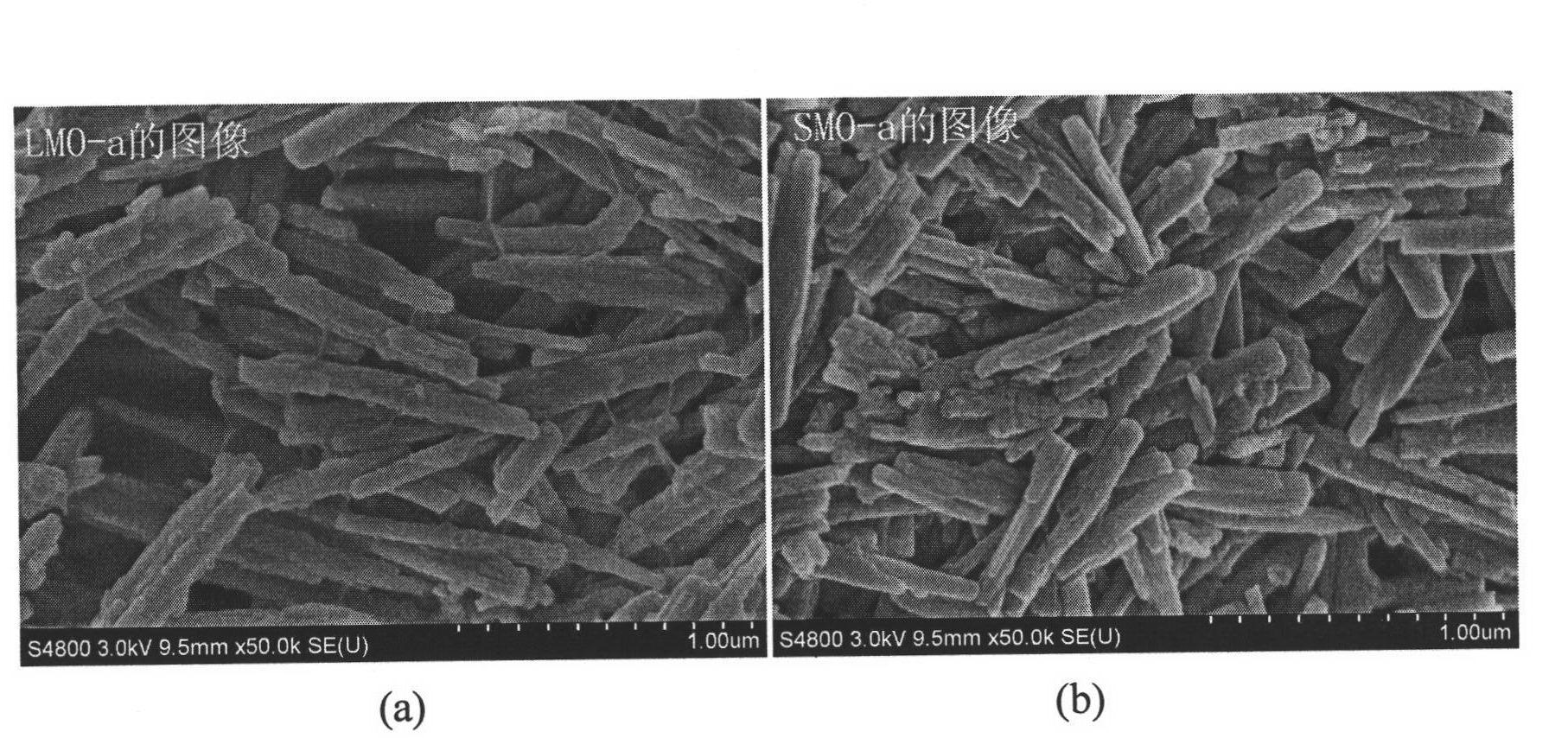
[0041] Dissolve 7.00g of acrylamide (monomer) and 0.14g of N,N-methylenebisacrylamide (crosslinker) in 20ml of deionized water, and add 7.00g of Li 4 mn 5 o 12 Powder, after stirring evenly, add 0.27g initiator ammonium persulfate, and quickly transfer it to the above-mentioned four-necked flask, stir vigorously, heat up to 65°C, N 2 Polymerize under atmosphere for 4 hours, cool to room temperature, wash the obtained particles with deionized water, and dry to obtain a granular ion sieve precursor (abbreviated as LMO-a). Use 1.0mol·l -1 HCl (H / Li=2, molar ratio) solution delithiates LMO-a, washes, and dries to obtain the target object (modified lithium ion sieve, abbreviated as SMO-a), with an average particle size of 1 mm to 2 mm. SEM image see figure 1 .
Embodiment 2
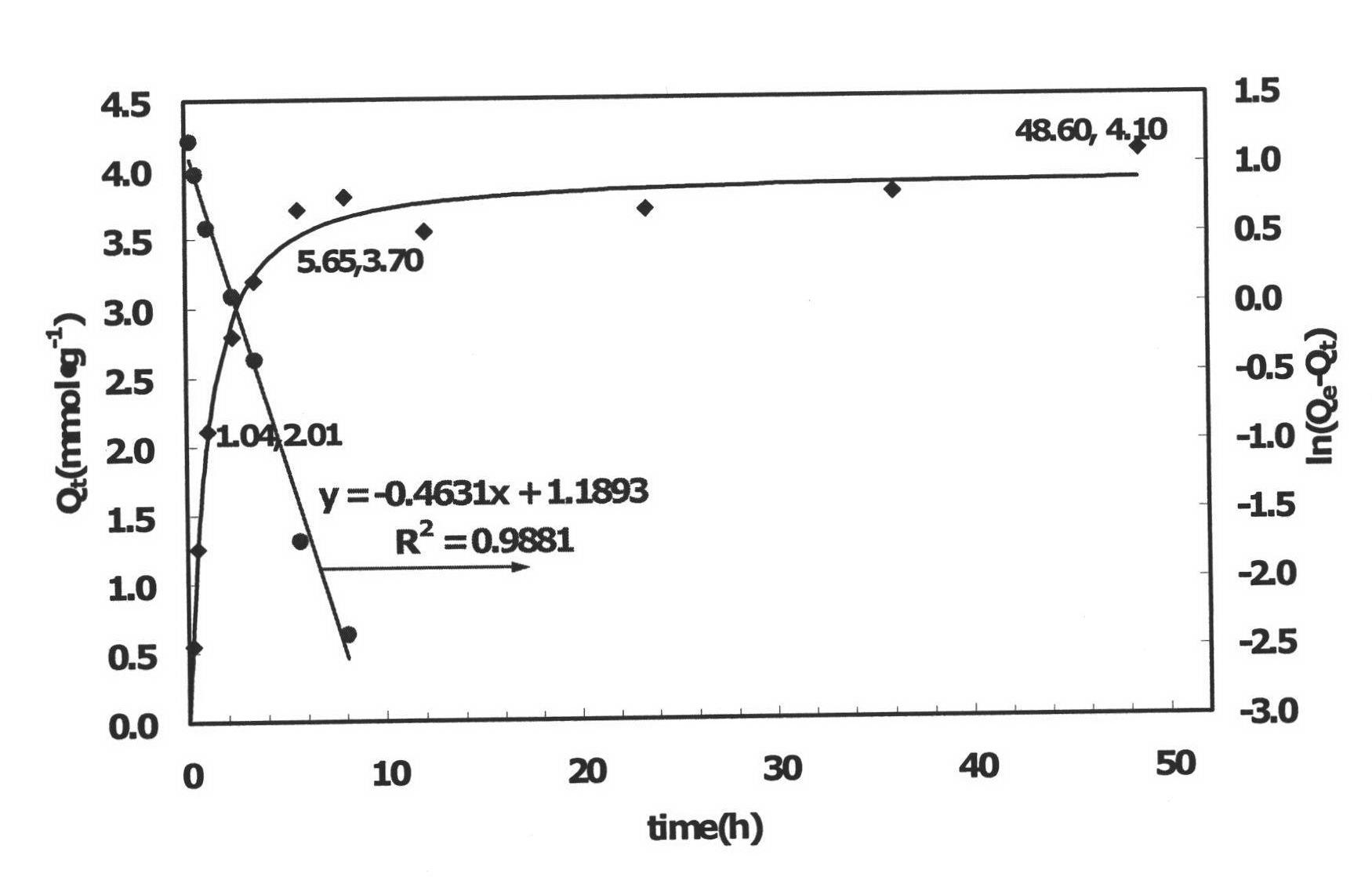
[0043] Weigh 0.2g of SMO-a respectively, and measure 0.010mol·1 -1 LiCl NH 3 ·H 2 O-NH 4 Cl buffer solution (pH value 9-11), mix the two and put them into a constant temperature oscillator for adsorption experiments. The adsorption temperature is 30°C and the rotation speed is 150r min -1 , respectively measure the concentration of lithium ions in the solution at different times, the results are shown in figure 2 .
[0044] Depend on figure 2 It can be seen that after SMO-a is adsorbed for 15 minutes, the adsorption amount is 0.54mmol g -1 , after 1.04 hours, the adsorption capacity exceeded 2mmol·g -1 , the saturated adsorption capacity is about 4.10mmol·g -1 . The adsorption rate constant k of the formed granular ion sieve SMO-a ads is 1.286×10 -4 the s -1 , in the same order of magnitude as the powdered ion sieve (the adsorption rate k of the powdered ion sieve ads 3.290×10 -4 the s -1 ). And the saturated adsorption capacity is equivalent to that of powder...
Embodiment 3
[0046] Equipped with reflux condenser, stirring device, N 2 Add 200ml hexanaphthene (oil phase) and 0.50gspan-80 (dispersant) in the four-neck flask of conduit, 20 ℃, N 2 Pre-mixed evenly under the atmosphere;
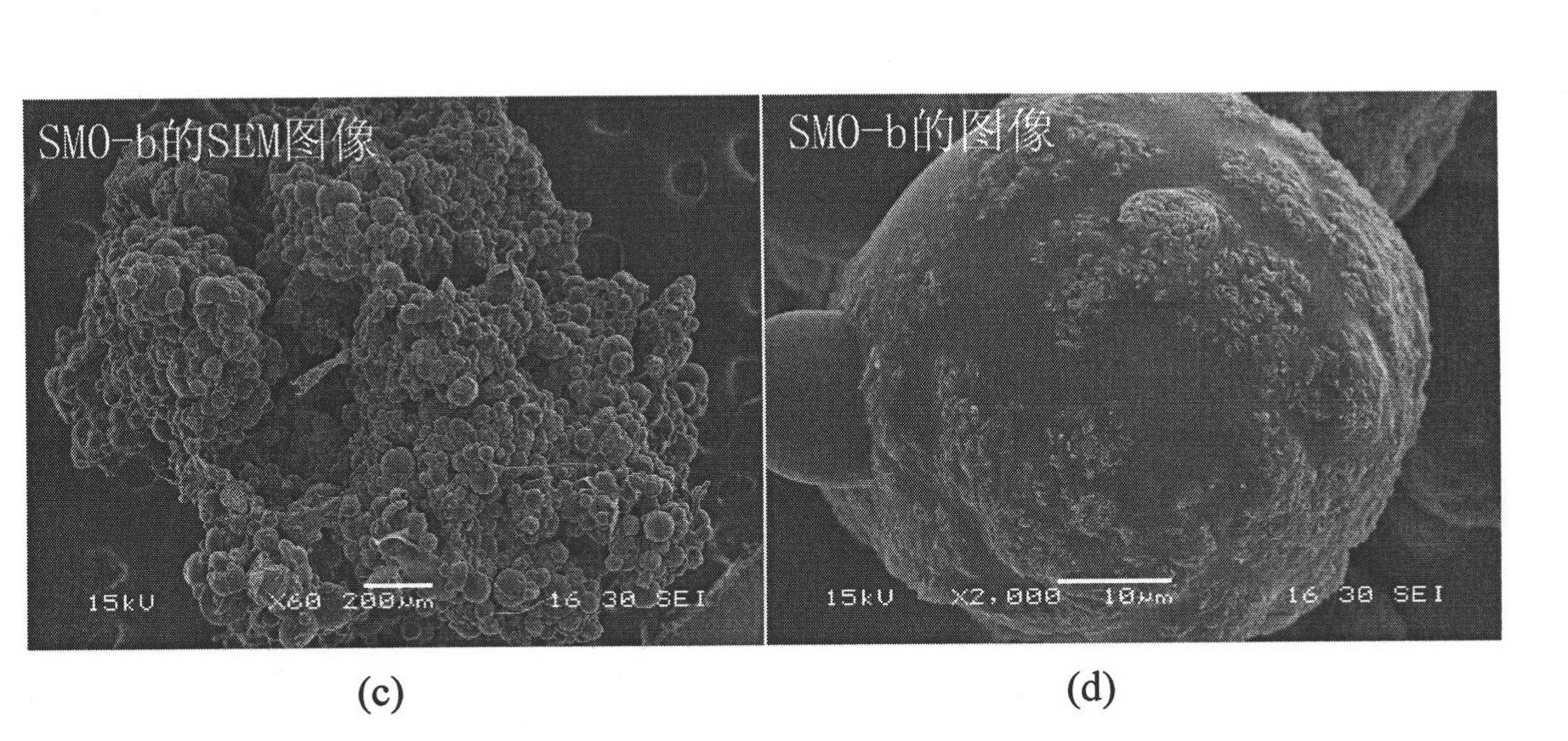
[0047] Dissolve 7.00g of acrylamide (monomer) and 0.14g of N,N-methylenebisacrylamide (crosslinker) in 10ml of deionized water, and add 14.00g of Li 4 mn 5 o 12 Powder, after stirring evenly, add 0.27g initiator potassium persulfate, and quickly transfer it to the above-mentioned four-necked flask, stir vigorously, and heat up to 70°C, N 2 Under the atmosphere, polymerize for 10 hours, cool to room temperature, and wash the obtained particles with deionized water completely, and dry to obtain a granular ion sieve precursor (abbreviated as LMO-b). -1 HCl solution delithiates LMO-b, washes, and dries to obtain the target object (abbreviated as SMO-b), whose average particle size is 1mm-2mm, and its SEM image is shown in image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com