Method for extracting alkali metal from salt lake brine and seawater through membrane extraction-back extraction
A technology of salt lake brine and alkali metal, applied in the direction of liquid solution solvent extraction, etc., can solve the problems of corrosion, long extraction process, and high requirements for experimental equipment, and achieve the effect of simple technical process, rich material and liquid resources, and overcoming instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
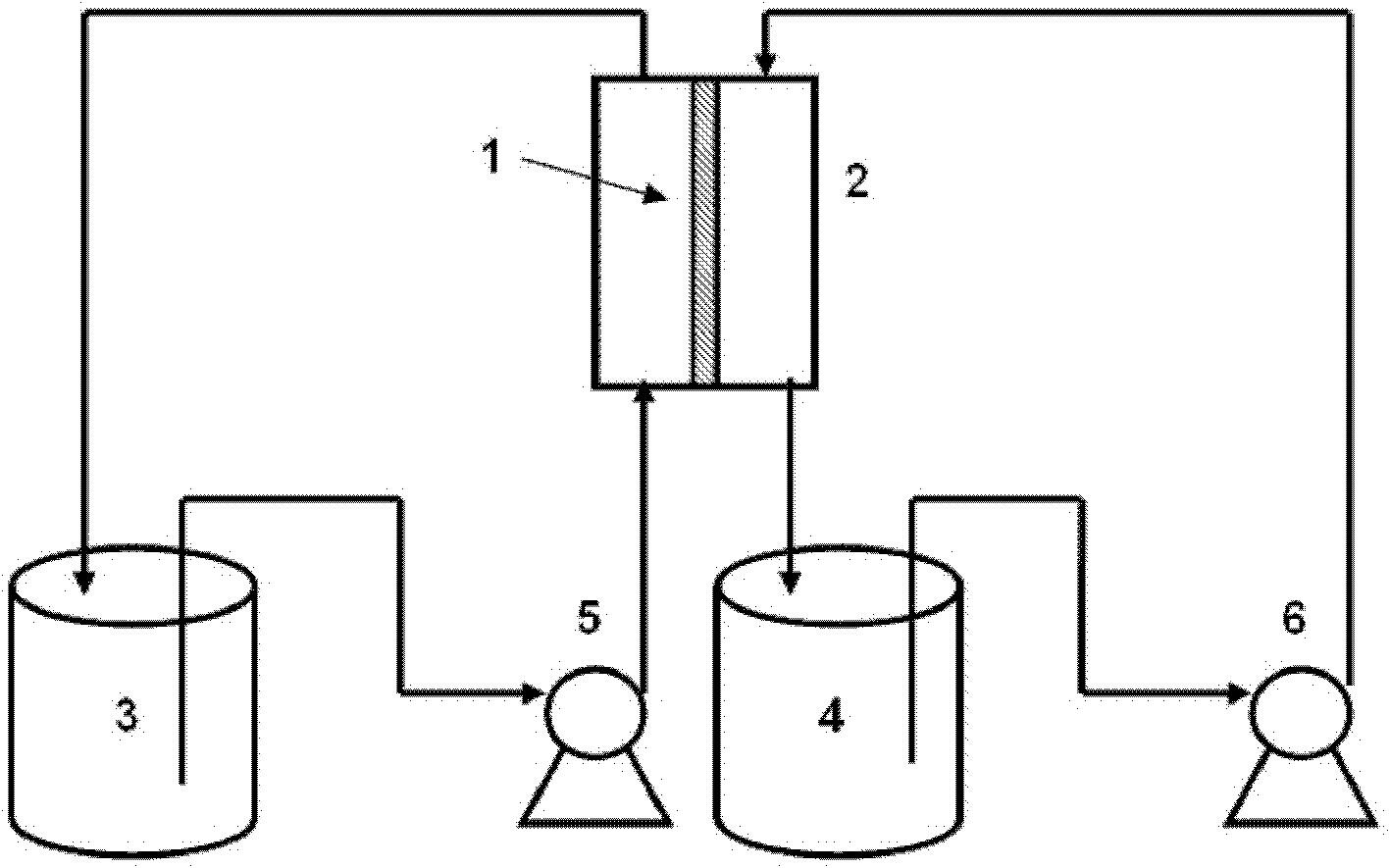
[0040] Lithium is extracted from salt lake brine through sun-dried saturated magnesium chloride deboronation mother liquor, and the mother liquor composition (g / L): Li + 1.5~2.4,Na + 1.8, Mg 2+ 100~140, Cl - 300,K + 1.4, SO 4 2- 20. Batch-type membrane extraction-re-extraction process is used to extract lithium, and the organic solution is composed of N,N-bis(1-methylheptyl)acetamide (N503) 10%-tributyl phosphate 20%, dissolved in n-octyl In alcohol, a sulfonated polyetheretherketone / polyethersulfone blended anion exchange membrane is fixed in a flat membrane module with a membrane area of 30cm 2 200mL of the above mother liquor and 100mL of the organic solution containing the extractant were sent to both sides of the ion exchange membrane respectively. The flow rate of the feed liquid was 3m / s, and the flow rate of the organic solution was 1m / s. The membrane extraction and membrane stripping experiments were carried out at 30°C. , Li in the feed liquid continuously pa...
Embodiment 2
[0044] Lithium is extracted from salt lake brine through sun-dried saturated magnesium chloride deboronation mother liquor, and the mother liquor composition (g / L): Li + 1.5~2.4,Na + 1.8, Mg 2+ 100~140, Cl - 300,K + 1.4, SO 4 2- 20. Use intermittent membrane extraction-back extraction process to separate lithium. First, take 200mL of the mother liquor and an equal volume of organic solution containing extractant in a grinding-mouth plugged separatory funnel. The organic solution is composed of diisobutyl ketone 80 %-Tributyl phosphate 20%, dissolved in No. 200 kerosene, fully shaken at room temperature for 10 minutes, static phase separation for about 30 minutes to completely separate the layers, and separate the organic solution containing the extractant that has been saturated with lithium. A sulfonated polyarylethersulfone ketone / polyethersulfone blended anion exchange membrane is fixed in a flat membrane module with a membrane area of 30cm 2 200mL of organic soluti...
Embodiment 3
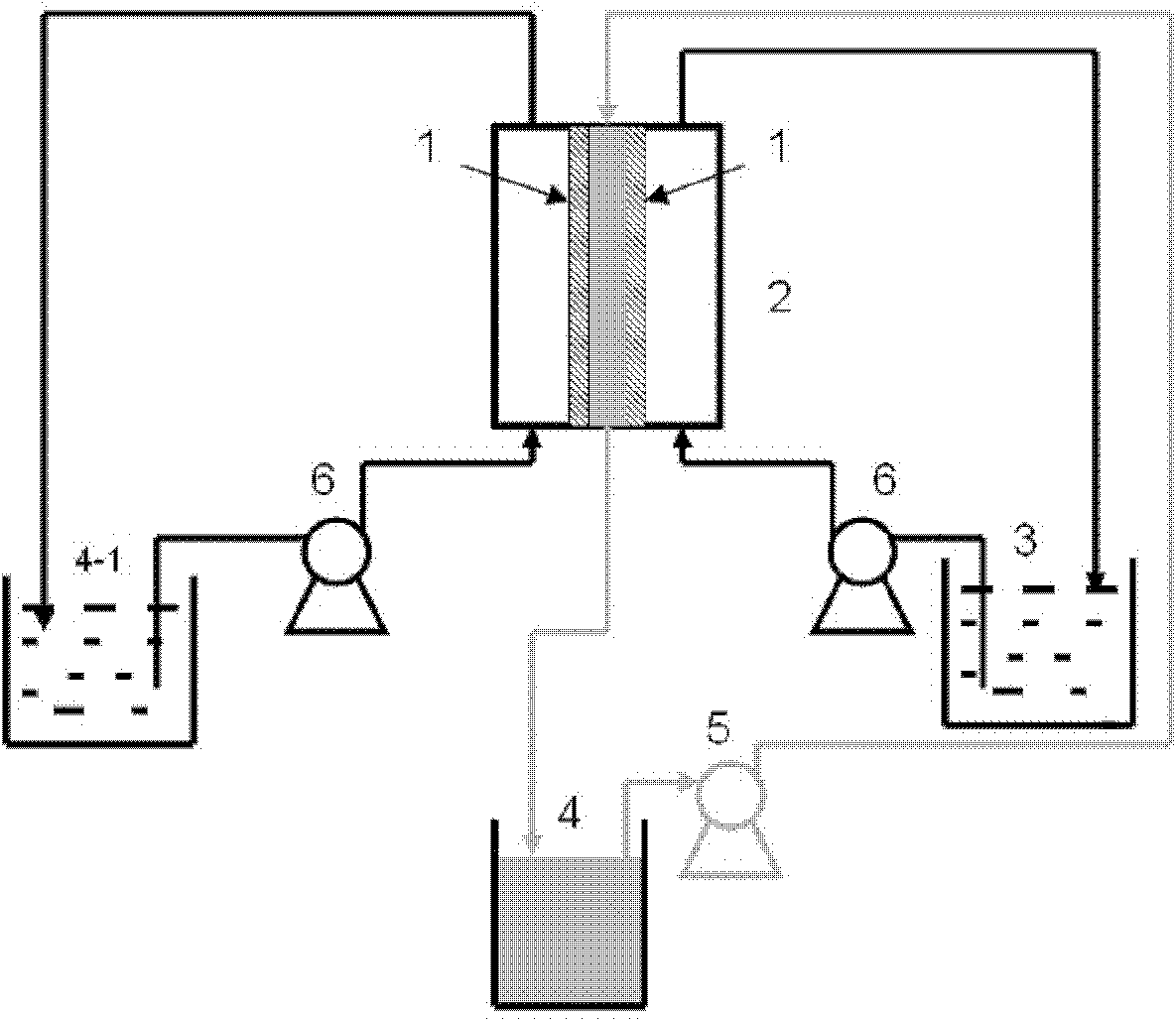
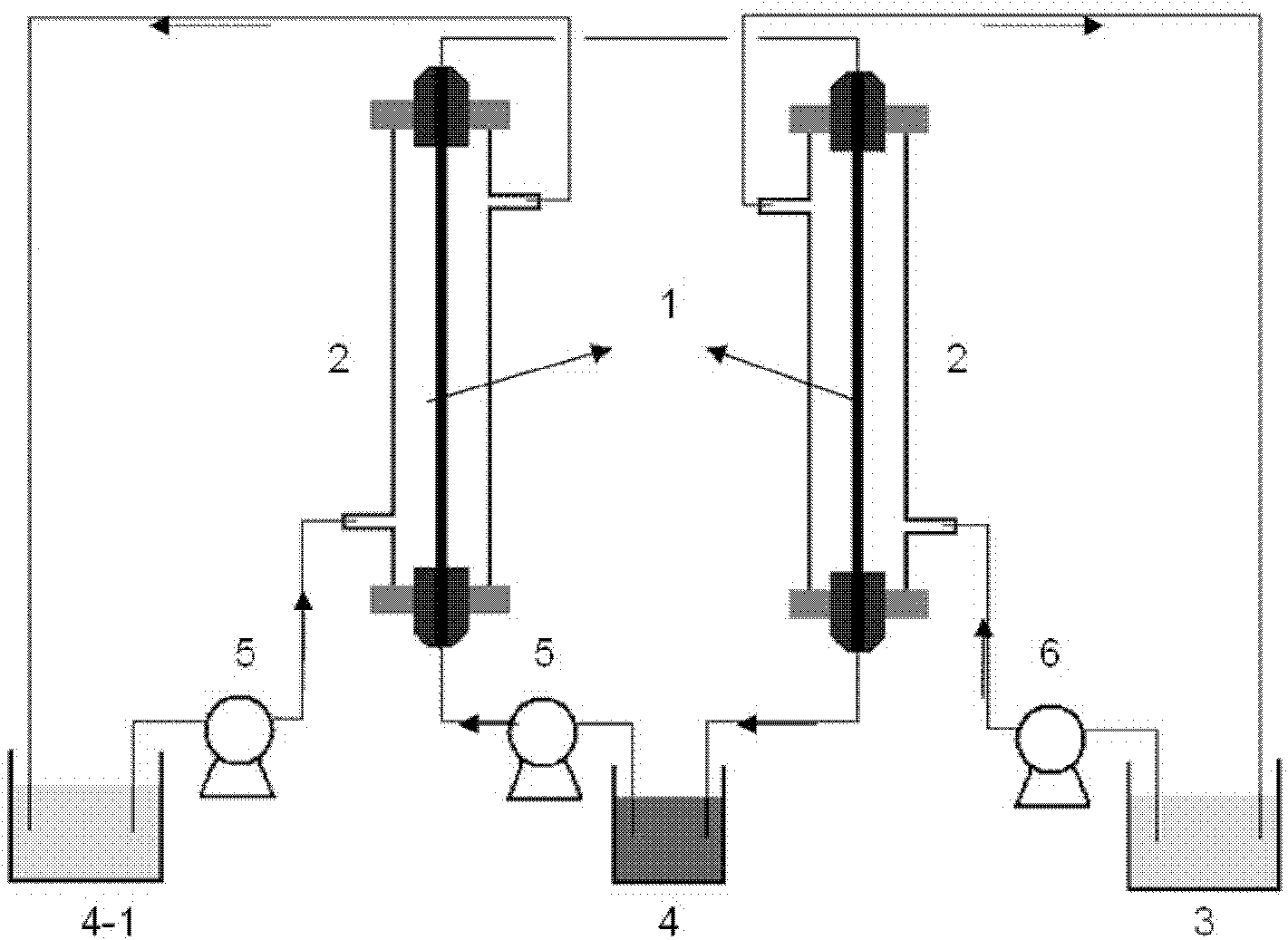
[0049] Lithium chloride-type salt lake brine concentrate, the composition after deboronization is shown in Table 1, and FeCl is added to the above-mentioned salt lake brine concentrate 3 ·6H 2 O 113g, so that the molar ratio of iron to lithium is 1.5, the color of the solution is reddish brown, and the pH is about 1. The continuous membrane extraction-back extraction process is used to separate lithium, and the temperature is constant at 25°C. The organic phase is composed of tributyl phosphate TBP 50%, diluent No. 260 solvent oil 50%, two sulfonated polyarylether sulfone ketone / polyether sulfone blended anion exchange membranes fixed in the plate and frame membrane module, the membrane area is 30cm 2 , 800mL of lithium chloride-type salt lake brine deboroning concentrate and 200mL of 6mol / L hydrochloric acid solution are sent to both sides of the two ion exchange membranes respectively, and are continuously circulated on both sides of the membrane at a speed of 4m / s and 3m / s....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
| External area | aaaaa | aaaaa |
| Area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com