Improved perfluorinated membranes and improved electrolytes for redox cells and batteries
A battery pack and electrolyte technology, applied in the direction of battery electrodes, electrode manufacturing, fuel cell components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
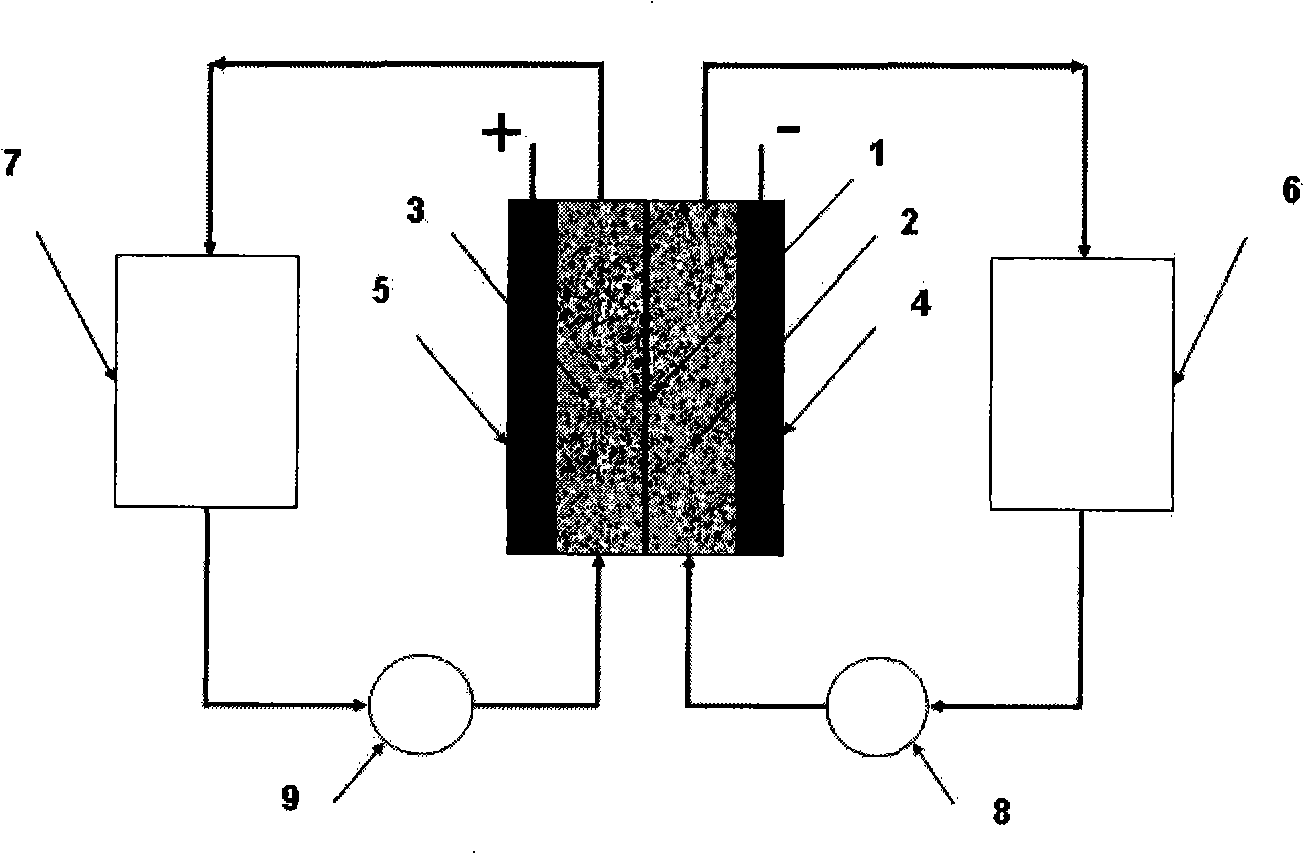
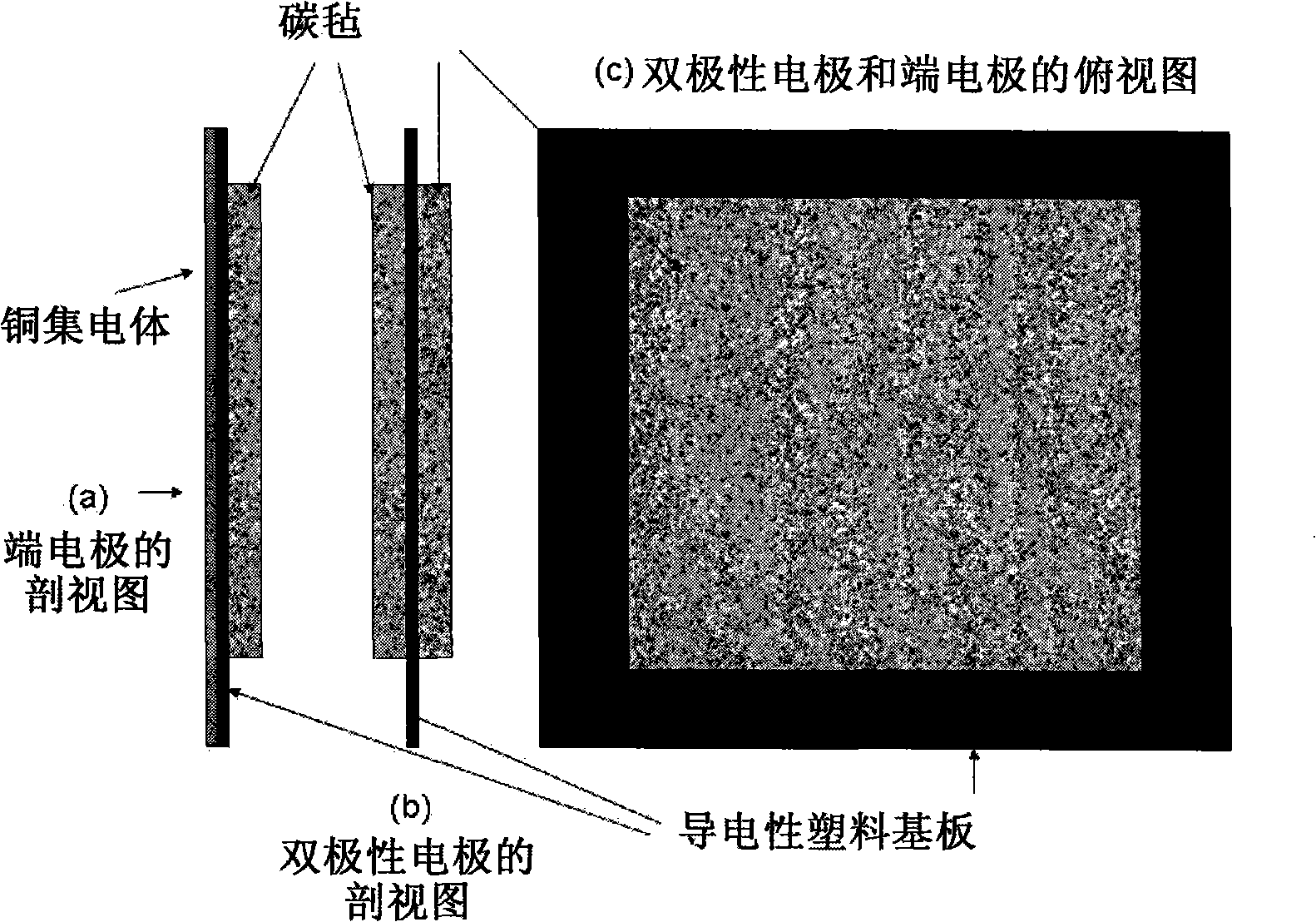
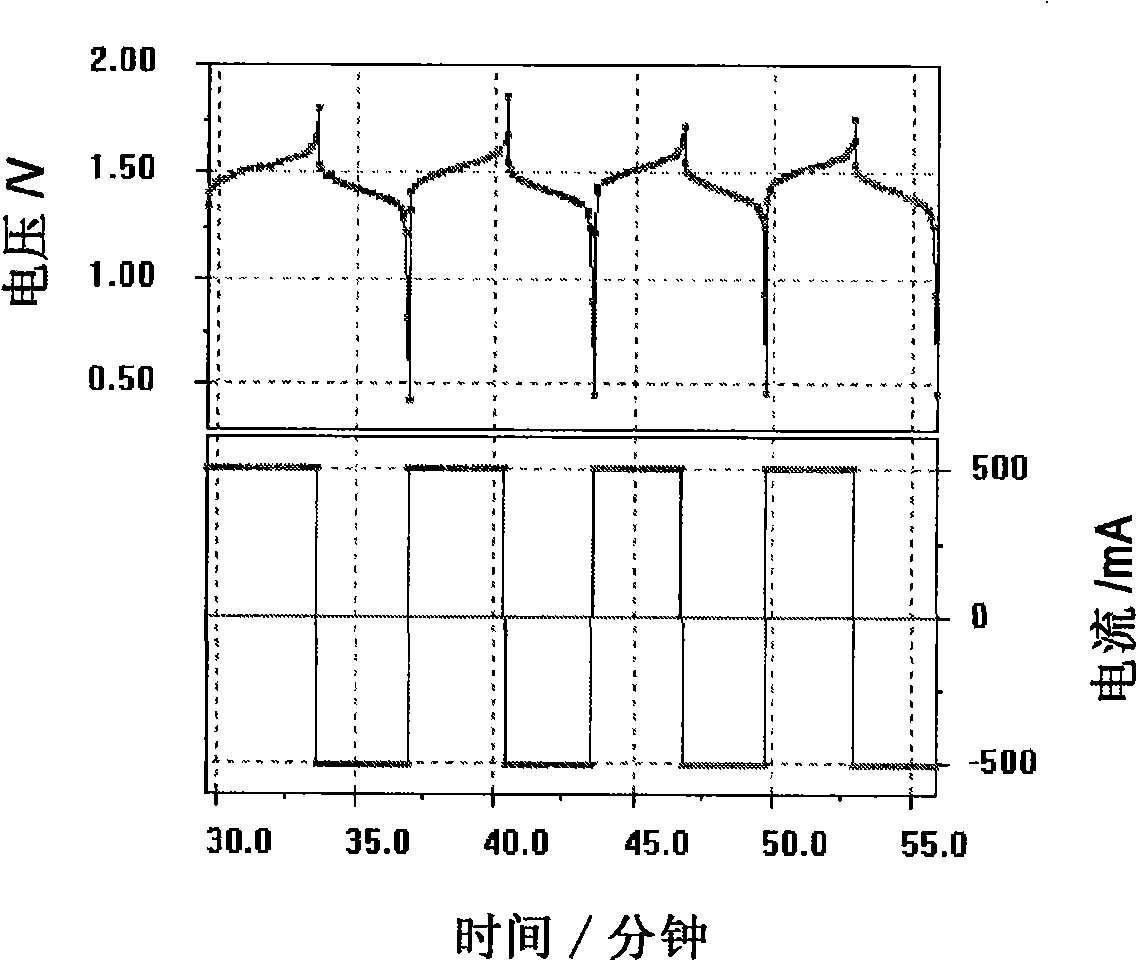
[0058] Two carbon felt / conductive plastic electrodes according to the invention were prepared by placing a conductive plastic sheet with a composition of 20% carbon black and 80% high-density polyethylene in a mould, and in the mould, , a piece of copper felt was placed on one side of the conductive plastic, and a 5 cm x 5 cm Sigri felt was placed on the opposite side. The mold was closed and heated to 240°C by placing it on a heated platen. A 125kg weight was placed on top of the mold for 15 minutes. The mold is then removed from the heated platen and allowed to cool to room temperature. Two terminal electrodes thus prepared were used in a vanadium redox cell. First dip the carbon felt in 5M H 2 SO 4 In a solution of 0.9M V(III) and 0.9M V(IV) in the medium, the electrode is placed between two flow frames with a cast-formed perfluorinated film (by sandwiched between two end plates). Cycling was performed with a battery charge and discharge current of 500mA.
[0059] Th...
Embodiment 2
[0061] A bipolar electrode of the present invention was prepared by placing 5 cm x 5 cm Sigri carbon felt on either side of the conductive plastic sheet of Example 1 in a mold and heating to 230°C. A 100 kg weight was placed on top of the mold for 15 minutes. The mold was then cooled to room temperature and the conductivity of the electrode was tested by placing it between two copper sheets and passing a current of 1 amp. After subtracting the voltage drop across the current collector, the recorded voltage drop for the bipolar electrode was 50 mV. Using Ohm's law and an electrode area of 25cm 2 Under the conditions, the resistivity of the bipolar electrode was determined to be 1.25 ohm cm 2 .
[0062] A second experiment was performed using the same temperature and pressure, except that the bonding time was increased to 20 minutes. The resistivity of the prepared bipolar electrode is about 1 ohm cm 2 .
Embodiment 3
[0064] Bipolar electrodes were prepared by the following steps: two carbon felts with a size of 400 mm × 400 mm were respectively placed on a conductive plastic (composed of 20% carbon black and 80% high carbon black) with a thickness of 1 mm and a size of 600 mm × 600 mm density polyethylene) on both sides of the sheet. By applying 2.5kg·cm at 230°C -2 The pressure was 15 minutes, and the carbon felt was thermally bonded to both sides of the conductive plastic sheet respectively. After cooling to room temperature, the conductivity of the resulting electrode was tested by placing 2 pieces of copper on both sides of the bipolar electrode and applying a current of 10 amps to the copper pieces. It was found that the conductivity of the bipolar electrode was 0.8 ohm cm 2 to 1.2 ohm·cm 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com