Pure titanium production from titanium monoxide/titanium carbide soluble solid anode electrolysis
An anode electrolysis and titanium monoxide technology, which is applied in the field of titanium monoxide/titanium carbide soluble solid solution anode electrolysis to produce pure titanium, can solve the problems of high anode slime, continuous electrolysis cannot be carried out normally, and the electrode process is hindered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Anode preparation
[0051] Raw materials: carbon powder, titanium dioxide are mixed in the following reaction stoichiometric ratio.
[0052]
[0053] Forming pressure
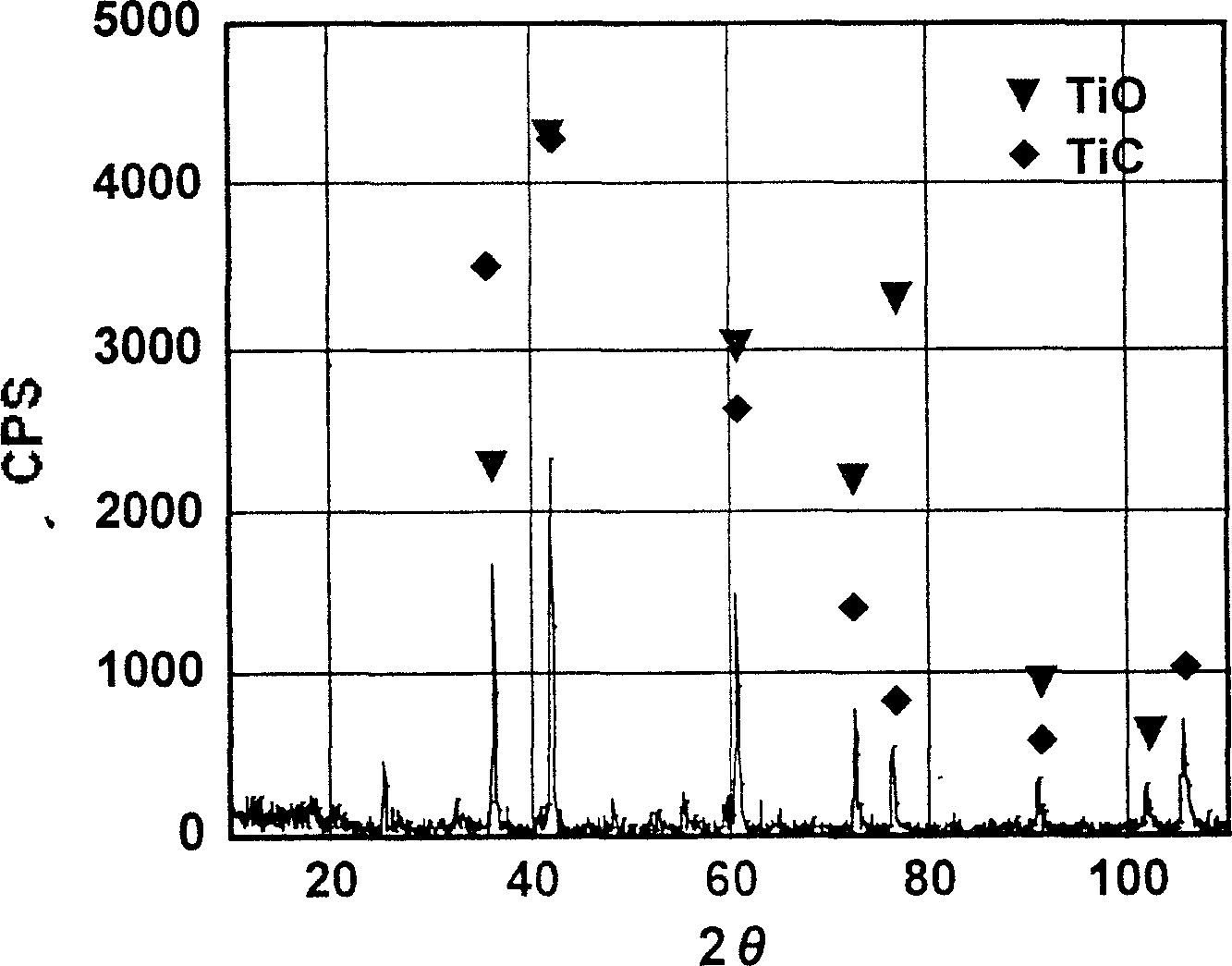
[0054] The resistance of the pressed block before heat treatment was 38 ohm·cm, and the resistance dropped sharply to 0.1 ohm·cm after heat treatment. The elemental analysis of the material after heat treatment shows that its atomic ratio should be Ti 2 CO, the structural composition of the material was analyzed by means of X-ray diffraction, and the results are shown in the attached figure 1 , it can be seen from the figure that the structure of the material changes significantly after heat treatment, mainly TiC·TiO solid solution with metal conductivity.
Embodiment 2
[0056] Anode preparation
[0057] Raw materials: Titanium carbide, titanium dioxide powder mixed in the following reaction stoichiometric ratio.
[0058]
[0059] Preparation process conditions (see the table below)
[0060] Forming pressure
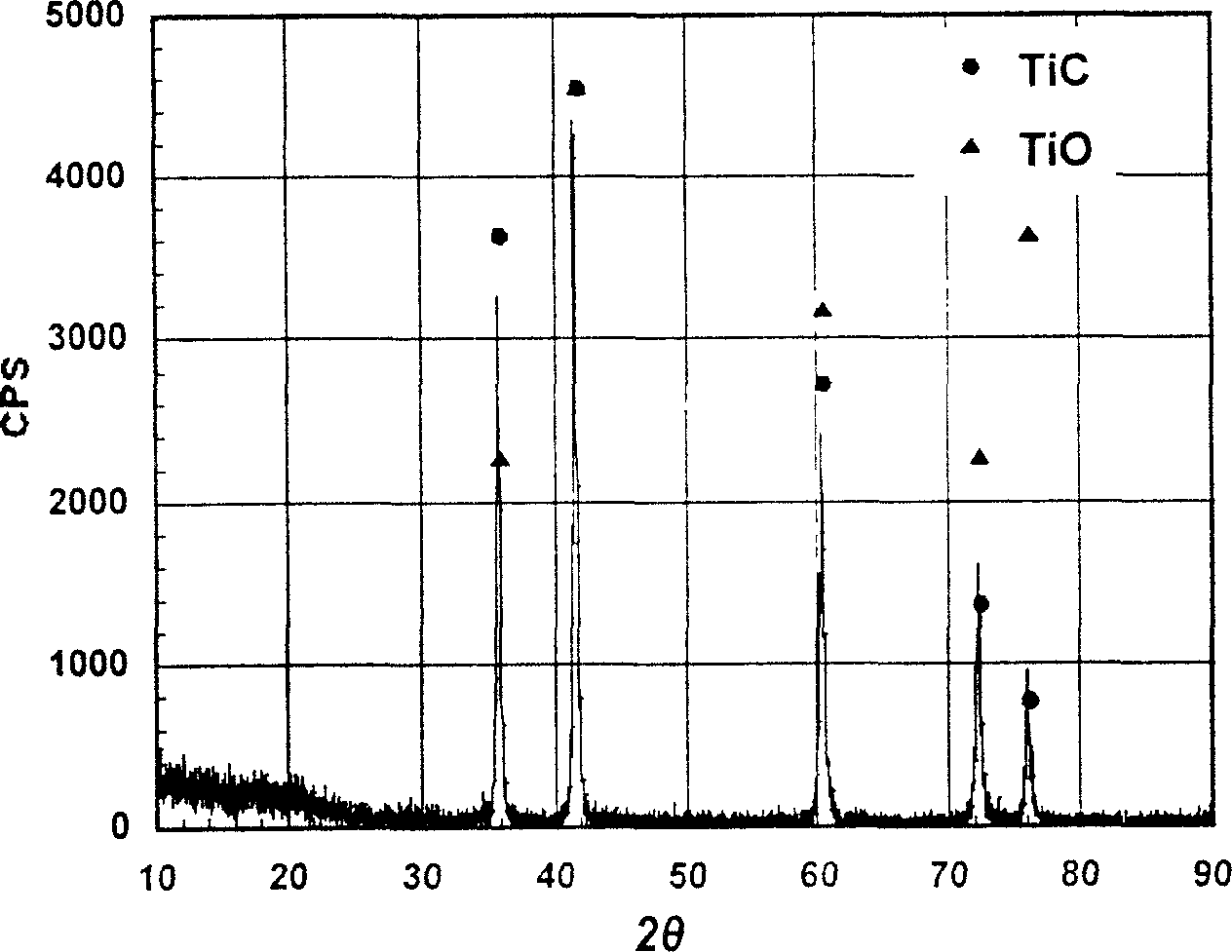
[0061] The resistance of the pressed block before heat treatment was 72 ohm·cm, and the resistance dropped sharply to 0.05 ohm·cm after heat treatment. The structural components of the material were analyzed by means of X-ray diffraction, and the results are shown in the appendix figure 2 , it can be seen from the figure that it is TiC·TiO solid solution after heat treatment.
Embodiment 3
[0063] temperature
[0064]
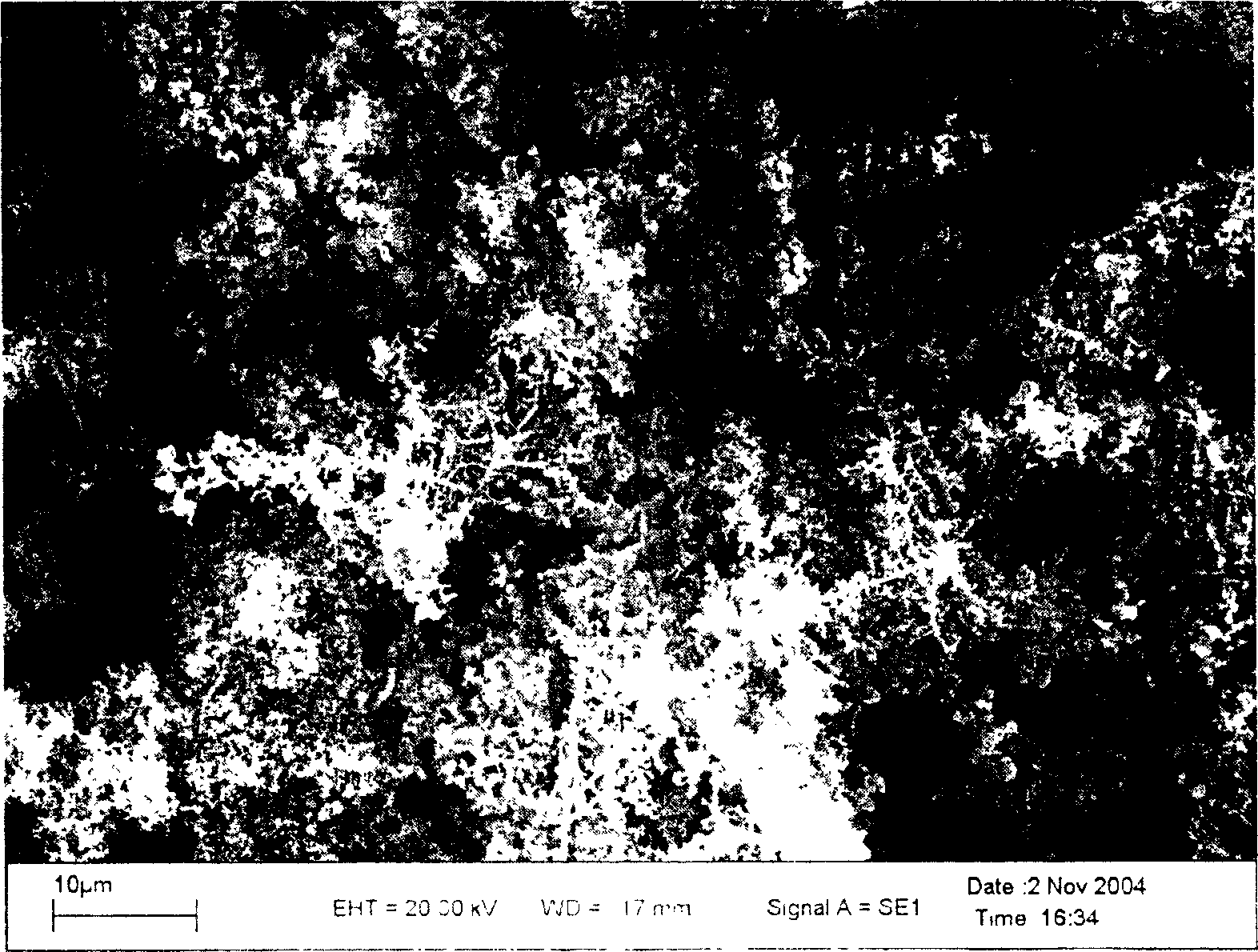
[0065] The data in the above table shows that carbon oxide gas is generated at the anode during the electrolysis process, and CO is the main form under the experimental conditions. After the electrolysis is completed, the cathodic deposition obtains pure titanium, which is washed with dilute hydrochloric acid (1wt%) and then washed with deionized water for 5 times and stored in natural drying. The cathode current efficiency calculated by Faraday's law is 90%; image 3 Scanning electron micrograph of the cathode product of Example 3. attached Figure 4Example 3 is an X-ray diffraction pattern of the cathode product, as can be seen from the figure, the cathode product has a crystal structure of pure titanium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com