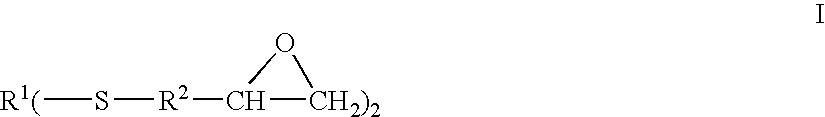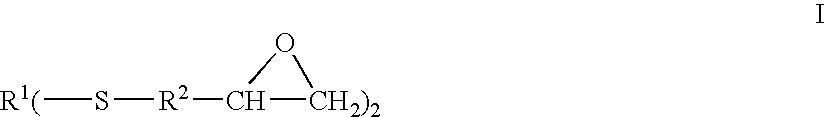Epoxy-capped polythioethers
a polythioether and epoxy-capped technology, applied in the field of epoxy-capped polythioethers and curable compositions, can solve the problem of extremely toxic epichlorohydrin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
253.4 g (1.39 mole) of dimercaptodioxaoctane (DMDO) was added to a 1 liter 4-neck flask under a nitrogen atmosphere. While stirring, the contents of the flask was heated to 50° C., and 146.6 g (0.93 mole) of diethylene glycol divinyl ether (DEG-DVE) was added over 1 hr. The temperature of the reaction mixture was increased to 70° C. and 0.05 g of free-radical initiator Vazo®67 (2,2′-azobis(2-methylbutyronitrile), Du Pont) was added. The temperature of the reaction mixture was maintained at 70° C. for an additional hour. Completion of the reaction of DEG-DVE with DMDO was indicated by a mercaptan equivalent value of 420. Allyl glycidyl ether (AGE) (110.87 g, 0.97 mole, 2% stoichiometric excess) was added at 70° C. over 1 hr and the reaction mixture was heated at 70° C. for an additional hour. Ten portions of Vazo®67 (0.165 g each) were then added at 3 hr intervals at 70° C. Following addition of Vazo®67 the reaction mixture was heated at 70° C. for 5 hr. The reaction mixture was the...
example 2
62.17 g (moles) of DMDO was added to a 250 ml 3-neck flask under a nitrogen atmosphere. While stirring, DMDO was heated to 60° C. and 44.88 g (mole) of DEG-DVE was added to the reaction mixture over a period of 50 minutes while the temperature of the reaction was maintained at 60° C.-70° C. The reaction mixture was heated at 70° C. for an additional 4 hr. Two portions of Vazo®67 (0.036 g each) were added to the reaction mixture at 1.5 hr intervals and heated at 70° C. for 1.5 hr. The mercaptan equivalent value of the reaction mixture was 890. An additional portion of Vazo®67 (0.036 g) was added and the reaction mixture heated for another 1.5 hr. A mercaptan equivalent value of 893 indicated completion of the reaction of DEG-DVE with DMDO. AGE (13.21 g, 0.116 mole, 2% stoichiometric excess) was added at 70° in one portion and the reaction mixture was heated for 2 hr. Eight portions of Vazo®67 (0.035 g each) were added at 3 hr intervals at 70° C. and heating was continued for another...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| hydrolysable | aaaaa | aaaaa |
| flexibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com