Double-layer sustained and controlled release nanoparticle and preparation method thereof and application
A nanoparticle and double-layer technology, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, and medical preparations containing active ingredients. Drug delivery carrier and other issues, to achieve the effect of long-term drug circulation, improve drug targeting, and reduce excretion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: Preparation of double-layer sustained and controlled release nanoparticles of the present invention
[0055] 1. Preparation of polyethylene glycol monomethyl ether grafted chitosan (mPEG-g-CS)
[0056] (1) Weigh 1 g of chitosan, add it to 10 mL of formic acid solution with a mass concentration of 1.0%, and stir to dissolve it;
[0057] (2) Add 3 g of polyethylene glycol monomethyl ether, stir for 15 minutes, then add 10 mL of formaldehyde solution with a mass concentration of 6.0%, and stir magnetically for 12 hours;
[0058] (3) Transfer to a dialysis bag, dialyze with deionized water for 3 days, and freeze-dry for 12 hours;
[0059] (4) Take the freeze-dried sample, wash it with chloroform, drain it, dissolve it with 0.1mol / L hydrochloric acid, put it into a dialysis bag again, dialyze it with deionized water for 3 days, and freeze-dry it for 24 hours to obtain a white cotton-like mPEG- g-CS sample.
[0060] 2. Preparation of mPEG-g-CS--PLGA bilayer c...
Embodiment 2
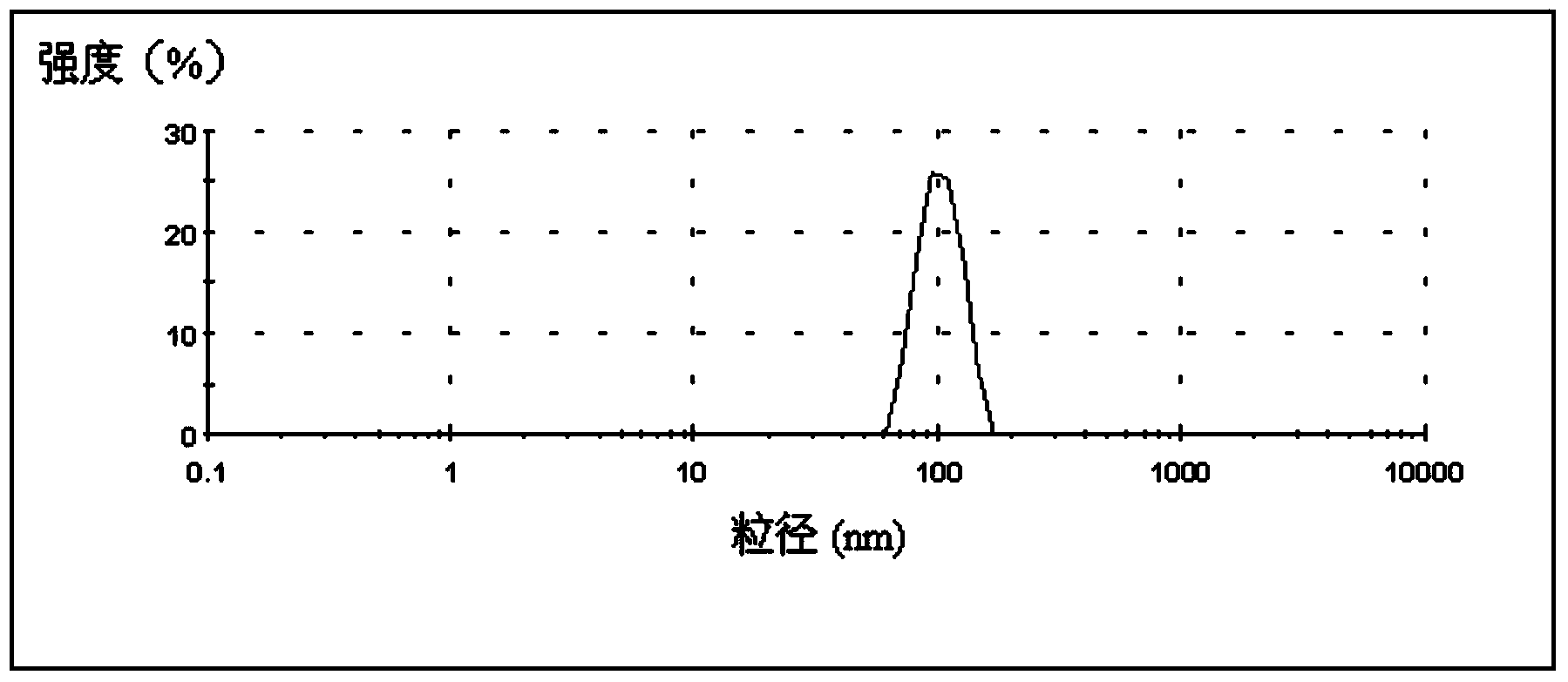
[0065] Embodiment 2: Performance determination of the double-layer sustained and controlled release nanoparticles of the present invention
[0066] 1. Determination of the polyethylene glycol grafting rate in mPEG-g-CS
[0067] Dissolve 50 mg of chitosan and 50 mg of mPEG-g-CS with dry constant weight in 25 mL of distilled water, respectively, and prepare sample solutions with a mass concentration of 0.02%; accurately pipette 5 mL of the above solution into a conical flask, and add 0.2 M 2mL of hydrochloric acid solution and a drop of toluidine blue indicator, shake well. Titrate with polyvinyl alcohol potassium sulfate standard solution until the sample solution turns from blue to bright purple, and at the same time take 5mL of distilled water for blank control test. The amino group content was calculated by the following formula (NH 2 %), from which the grafting rate of polyethylene glycol was derived.
[0068] NH 2 % ...
Embodiment 3
[0083] Example 3: Preparation of double-layer sustained and controlled release drug-loaded nanoparticles of the present invention
[0084] (1) Dissolve 100mg of PLGA in 4mL of dichloromethane, add 0.5mL of vincristine sulfate aqueous solution with a concentration of 20mg / mL, and ultrasonically emulsify for 2 minutes under 60W power to form W1 / O type colostrum;
[0085] (2) Stir the W1 / O type colostrum prepared in step (1) into an aqueous polyvinyl alcohol solution with a mass concentration of 1.5% (containing Tween 20 with a mass concentration of 1%), and continue stirring at 200rpm for 4 Hours, form W1 / O / W2 type double emulsion, volatilize organic solvent to solidify, to PLGA nanoparticle solution;
[0086] (3) Take the PLGA nanoparticle solution prepared in step (2), filter it with a 0.8 μm filter membrane, collect the filtrate, centrifuge at a speed of 18000 rpm, collect the nanoparticles, and freeze-dry for 12 hours to obtain solid powder PLGA nanoparticle grain;
[0087...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com