Paclitaxel mixed composition and water-in-oil type emulsion formulation for chemoembolization and preparation method thereof
A composition, paclitaxel technology, applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1. the manufacture of super liquefied lipiodol / iobemol / paclitaxel emulsion
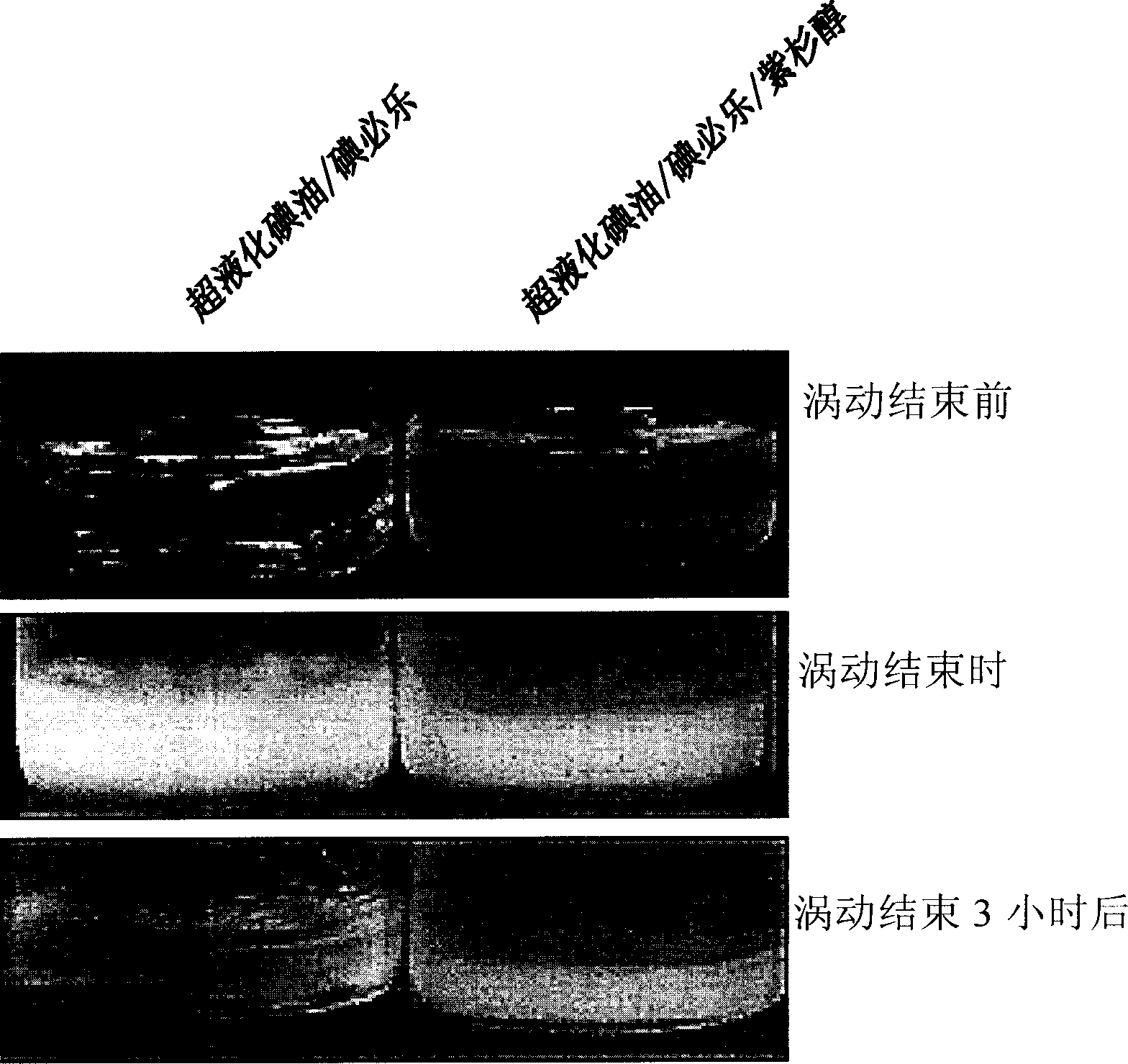
[0050] 800 μl of lipiodol Ultra-fluid (Lipiodol Ultra-fluid, Laboratoire Guerbet, France, iodine content of 38% by weight) was used as an oily contrast medium. Add the paclitaxel (Samyang Genex, Korea) of 8mg in the described super liquefied lipiodol, pack into test tube (micro test tube with safety plug, polyethylene system, 1.5ml, Eppendorf, Germany AG), stirred at room temperature to dissolve. It was heated to 40°C for rapid dissolution. To this oily mixture was added 200 μl of Iopexol (Bracco s.p.a. Italy). As a control mixture, a mixture of 0.8 ml of paclitaxel and 0.2 ml of iopexol was used. Photographs of these two mixtures after they were made are shown in figure 1 (upstream). Using a vortex mixer, each mixture was vortexed at maximum setting (120V, 0.65A, 60Hz) for 10 minutes. At the end of the vortexing, the lipiodol / iobiram / paclitaxel formulation did not phase-separa...
Embodiment 2
[0051] Embodiment 2. the manufacture of super liquefied lipiodol / iobix / paclitaxel / doxorubicin emulsion
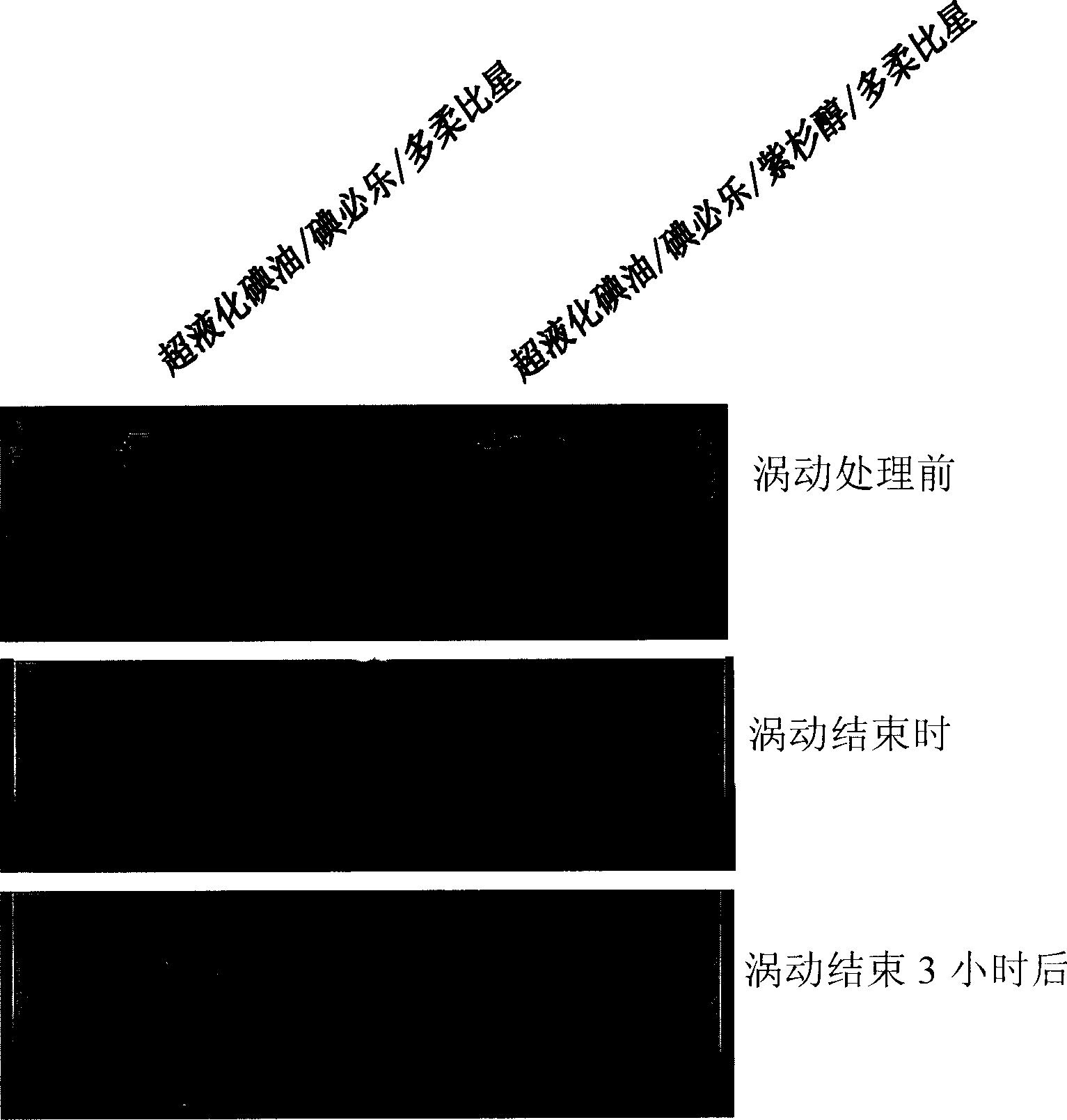
[0052]800 μl of lipiodol Ultra-fluid (Lipiodol Ultra-fluid, Laboratoire Guerbet, France, iodine content of 38% by weight) was used as an oily contrast medium. Add the paclitaxel (Samyang Genex, Korea) of 8mg in the described super liquefied lipiodol, pack into test tube (micro test tube with safety plug, polyethylene system, 1.5ml, Eppendorf, Germany AG), stirred at room temperature to dissolve. For rapid dissolution, sonicate with a tank sonicator. To this oily mixture was added 200 μl of iopexate (Bracco s.p.a. Italy) containing 4 mg of doxorubicin (Sigma Chemicals). As a control mixture, a mixture consisting of 0.8 ml of paclitaxel and 0.2 ml of iopexate containing 4 mg of doxorubicin was used. Photographs of these two mixtures after they were made are shown in figure 2 (upstream). Using a vortex mixer, each mixture was vortexed at maximum setting (120V, 0.65A, 60H...
Embodiment 3
[0053] Embodiment 3. the manufacture of super liquefied lipiodol / iobix / paclitaxel / carboplatin composition
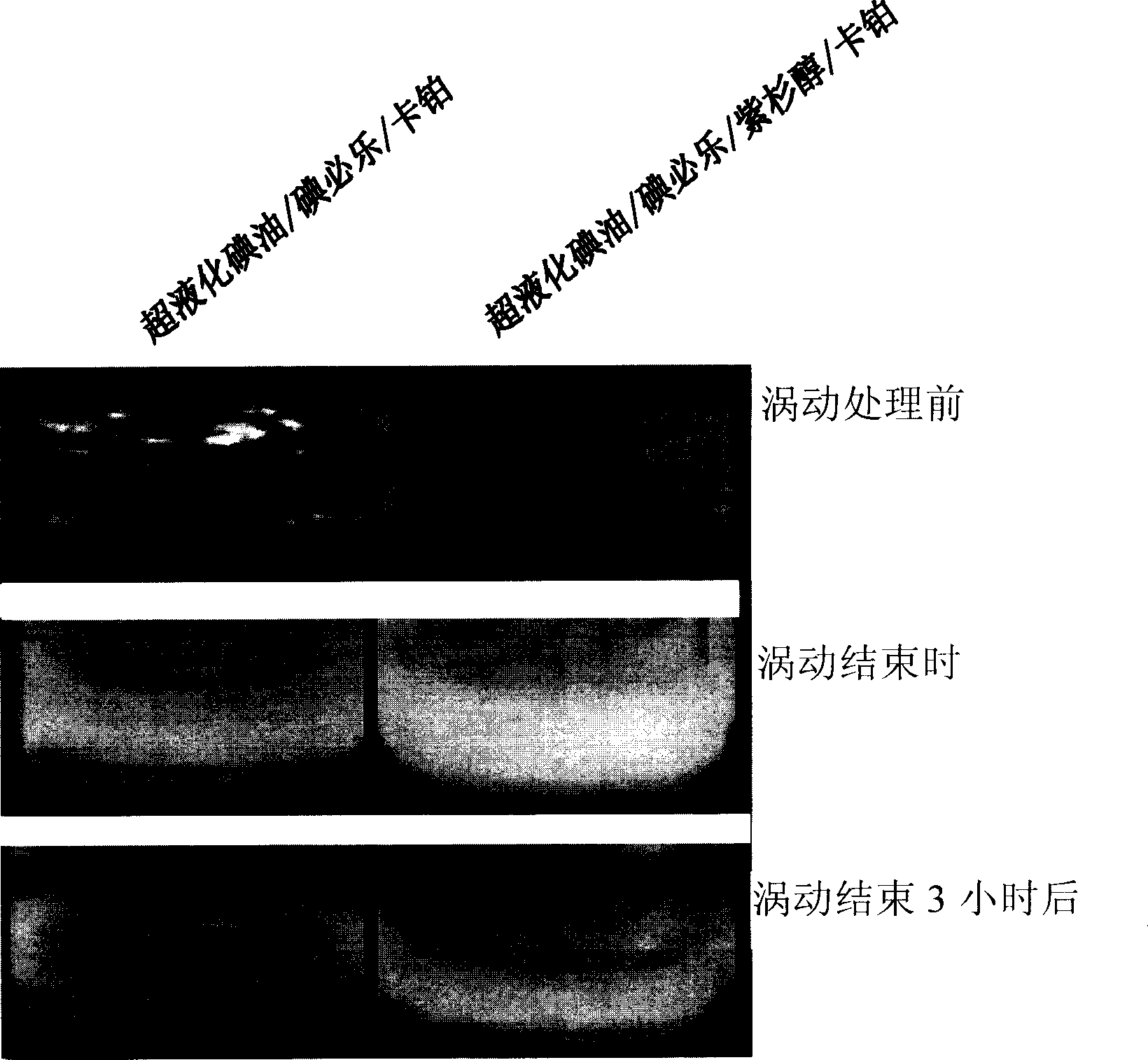
[0054] Compositions and controls were produced as in Example 2, except that 4 mg of carboplatin was used instead of doxorubicin. Photographs of these two mixtures after they were made are shown in image 3 (upstream). Using a vortex mixer, each mixture was vortexed for 10 minutes at maximum setting (120R, 0.65A, 60Hz). At the end of the vortexing, the lipiodol / iopix / paclitaxel / carboplatin formulation did not phase-separate to form an emulsion, while phase separation was observed in the lipiodol / iobix / carboplatin composition (BOC) . After 3 hours from the end of vortexing, the emulsion of the lipiodol / iopix / paclitaxel / carboplatin composition was stable, while the lipiodol / iobix / carboplatin composition was almost completely phase-separated (downstream). In the manufactured super liquefied lipiodol / iobixate / paclitaxel / carboplatin composition, only small water droplets (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com