VEGF receptor fusion protein and its use in preparation of medicament for treating eye disease
A fusion protein and receptor technology, applied in sensory diseases, drug combination, drug delivery, etc., can solve problems such as retinal scarring, detachment, and vision loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
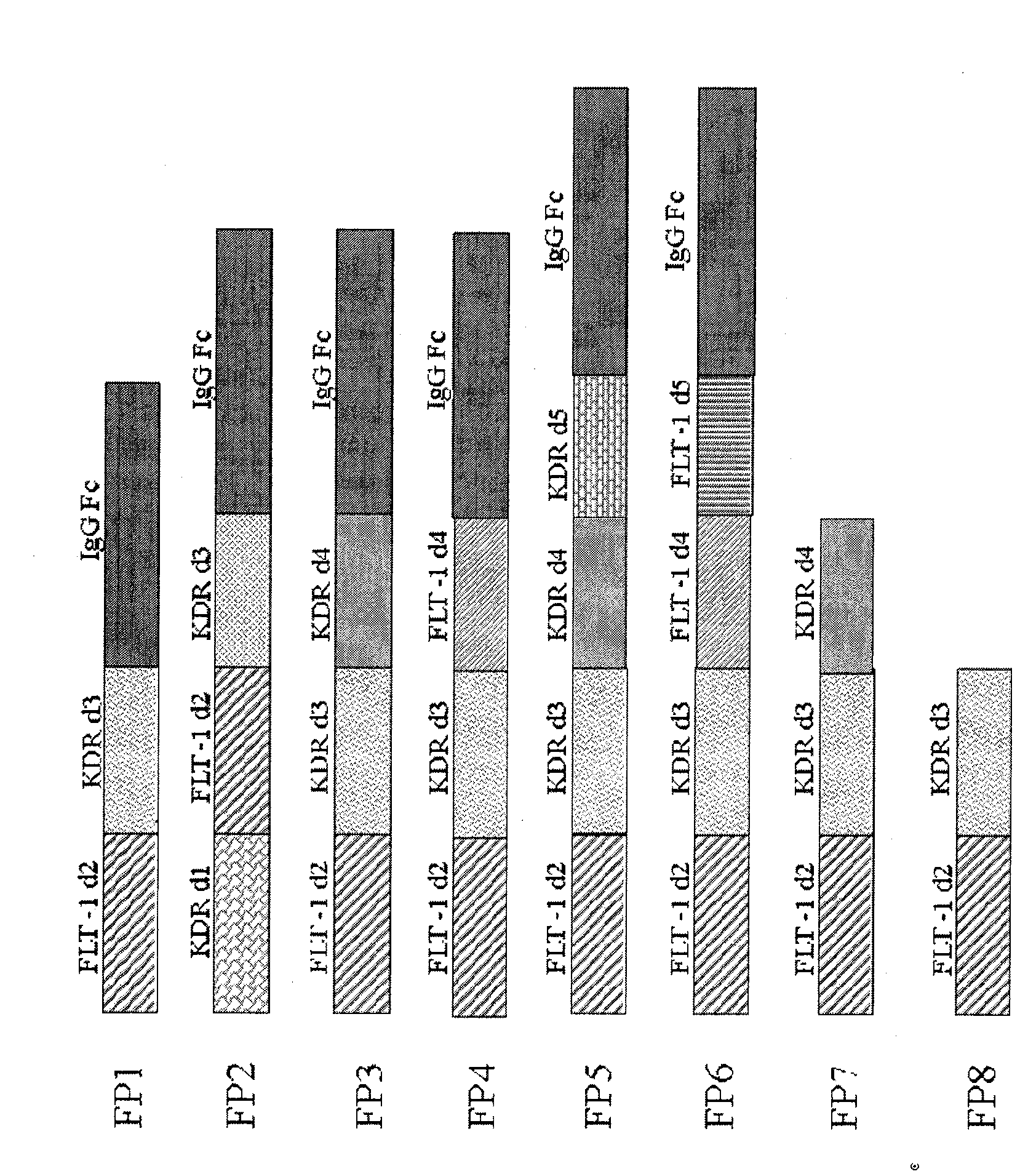
[0018] Example 1, FP 7 build
[0019] The fusion protein FP7 is extracted from HUVEC cells by primers flt-1D2(F), flt-1D2(R), KDR D3(F) and KDRD3-4(R) (see patent application, application number CN200510073595.4) The flt-1 and KDR gene fragments amplified on the cDNA synthesized by mRNA as a template are recombined. The specific conditions are denaturation at 95° for 30 minutes, annealing at 56° for 45 seconds, extension at 72° for 2 minutes, and PCR amplification for 30 cycles to obtain flt-1 and KDR IgG-like domains. PCR product. Using TA cloning kit, clone the PCR product into PCR2.1 plasmid, and transfect E.coli, select white colonies, add LB medium, and culture overnight. Plasmids were extracted with Qiangen plasmid extraction kit, digested and identified by sequencing. The splicing PCR (Sewing PCR) method is used to connect the flt-1 fragment, the KDR fragment and the ribonucleic acid of the partial sequence of the IgG hinge region. Design EcoR1 restriction sites in...
Embodiment 2
[0020] Example 2, FP 8 build
[0021]The fusion protein FP8 was directly amplified by PCR using FP7 as a template, and the primers used in PCR were flt-1D2 (F) and KDR D3-hing (R). The sequence of the latter is: 5'-aggtgctgggcacagtgggcatgtgtgagttttgtctttttcatggaccctgacaaatg. It includes a sequence complementary to the third immunoglobulin-like region of KDR and a partial nucleotide sequence of the human IgG Fc hinge region. The method of PCR amplification and gene cloning is identical with example 1. Finally, the PcDNA3.1 plasmid inserted with FP8 was transfected into CHO cells, and a stable cell line was obtained for protein expression. The amino acid sequence of FP8 is shown in Sequence List 2, and the nucleotide sequence is shown in Sequence List 4.
Embodiment 3
[0022] Example 3 Fusion protein and VEGF binding affinity experiments
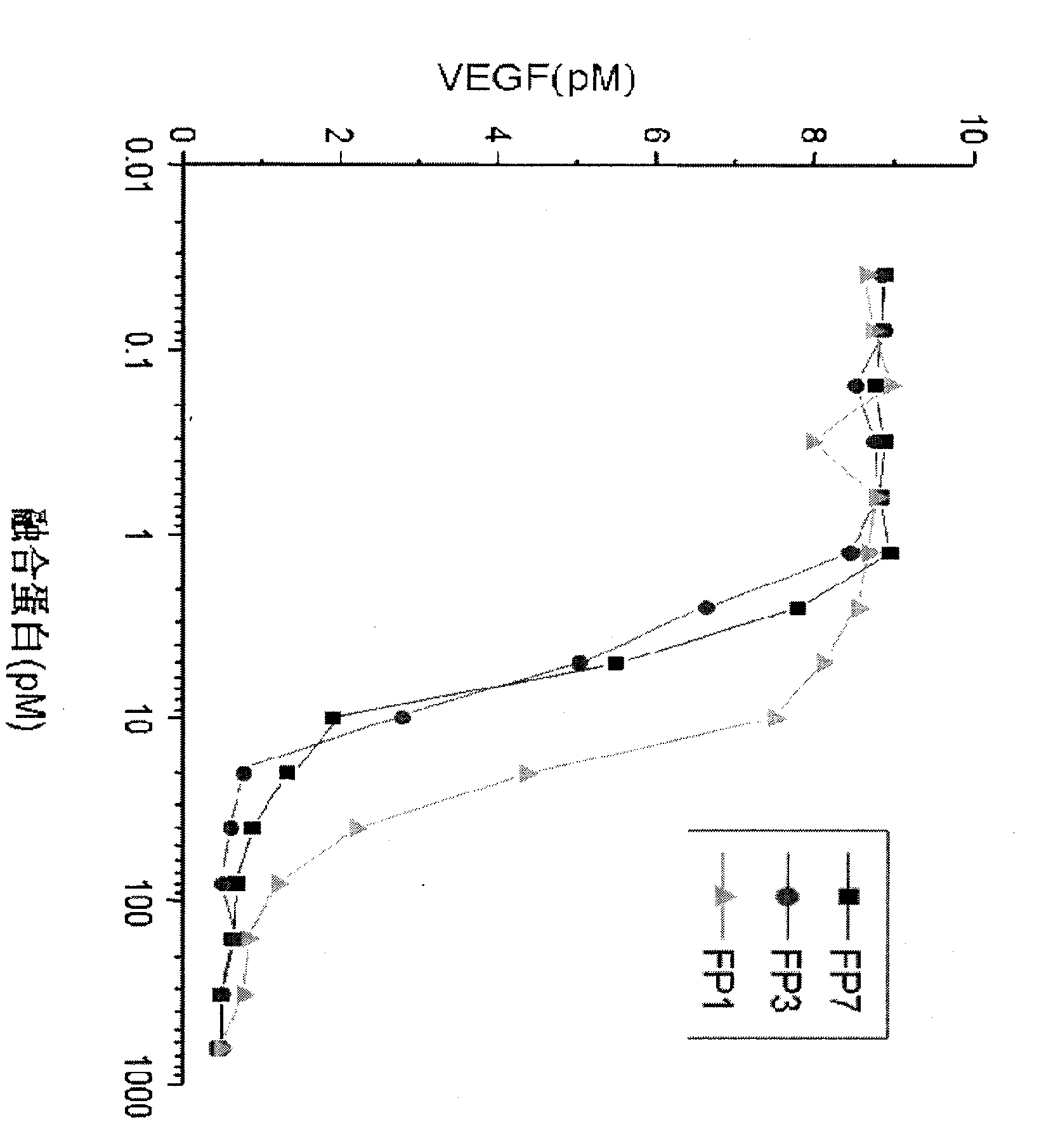
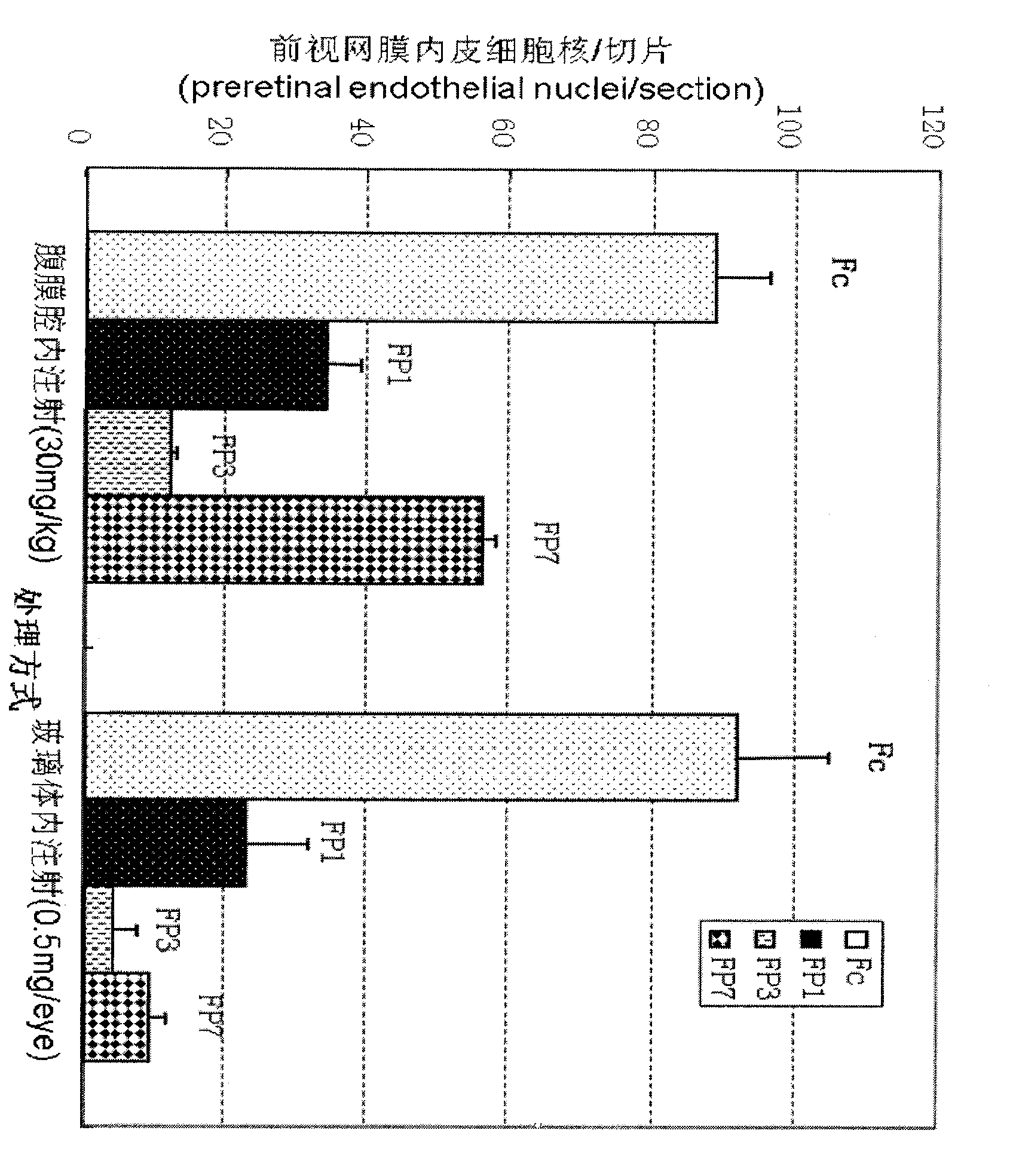
[0023] The present invention determines the ability of various fusion proteins to bind VEGF by measuring the amount of VEGF. In this test, a certain amount of VEGF (10PM) was added to the test tube, and then diluted various fusion proteins containing different amounts were added to the test tube containing VEGF, and after mixing, they were placed in a 37 ° incubator. Save for an hour. One hour later, the free VEGF in the test tube was measured by a kit for detecting the amount of VEGF provided by R&D systems——VEGF assay Kit. The measured results are processed by the software and obtained as follows: figure 2 the result of, figure 2 show that FP 1 , FP 3 and FP 7 can effectively bind to VEGF affinity, and its binding affinity can be determined by IC 50 Said, respectively, are 11.2PM, 4.3PM and 4.1PM. This experiment proves that FP 3 and FP 7 Binding ability to VEGF is similar in vitro, and both ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com