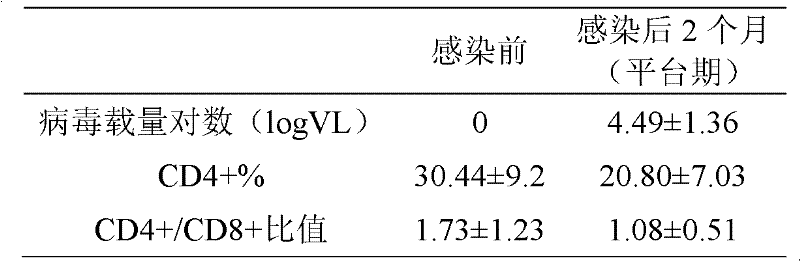
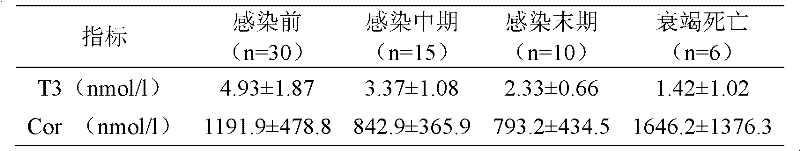
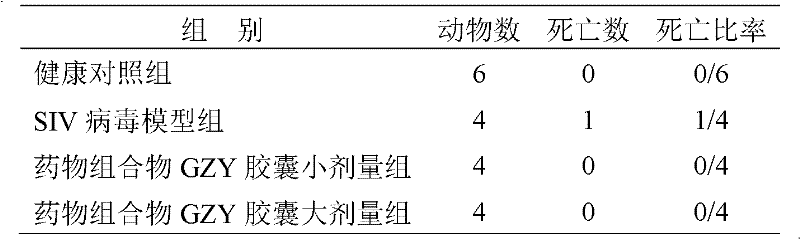
Medicinal composition for treating acquired immune deficiency syndrome (Aids) as well as preparation method, quality control method and application thereof
A composition and drug technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antiviral agents, etc., can solve problems such as the curative effect needs to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of above-mentioned pharmaceutical composition, comprises the steps:
[0044] 1) Take the crude drug, decoct it with water, filter, and take the filtrate;
[0045] 2) centrifuging the filtrate, taking the supernatant, concentrating, and extracting the concentrate with water;
[0046] 3) adding ethanol to the concentrated water extract, stirring, standing still, and then centrifuging, taking the supernatant, removing ethanol, and obtaining the ethanol extract;
[0047] 4) Prepare the pharmaceutical preparation by combining the alcohol extract and other acceptable pharmaceutical excipients.
[0048] Furthermore, its preparation method includes the following steps:
[0049] 1) Take the raw material drug, add water to decoct 3 times, add 20 times the amount of water each time, decoct for 2 hours, let cool, filter, and combine the filtrate;
[0050] 2) Centrifuge the filtrate, take the supernatant and concentrate it to the weight of the raw material ...
Embodiment 1
[0072] Raw material composition:
[0073] 25 parts of cooked aconite, 12.5 parts of epimedium, 12.5 parts of dried ginger, 12.5 parts of licorice, 7.82 parts of red ginseng, 7.81 parts of poria cocos, 7.81 parts of knotweed, 7.81 parts of salvia miltiorrhiza, 3.75 parts of Phellodendron phellodendri, and 2.5 parts of skullcap.
Embodiment 2
[0075] Raw material composition:
[0076] 20 parts of cooked aconite, 14 parts of epimedium, 15 parts of dried ginger, 10 parts of licorice, 10 parts of red ginseng, 12 parts of poria cocos, 6 parts of knotweed, 10 parts of salvia miltiorrhiza, 5 parts of Phellodendron phellodendri, and 3 parts of skullcap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com