Composite tablet of valsartan and hydrochlorothiazide and preparation method thereof
A technology of hydrochlorothiazide and composition, applied in the field of valsartan and hydrochlorothiazide pharmaceutical composition tablet and preparation field thereof, can solve the problem that the activity of soybean phosphatidylcholineylserine is not disclosed, and achieve liver protection, strong absorption capacity and effect Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1 Investigation on the preparation method of a pharmaceutical composition tablet of valsartan and hydrochlorothiazide
[0012] Research has proven that valsartan is a white powder; odorless and tasteless. Soluble in methanol, soluble in chloroform, almost insoluble in water; hydrochlorothiazide is a white crystalline powder; odorless, slightly bitter taste. This product is soluble in acetone, slightly soluble in ethanol, insoluble in water, chloroform or ether; soluble in sodium hydroxide solution; phosphatidylcholine is odorless or slightly nutty. Unstable in air and light, insoluble in water, hardly soluble in acetone, acetate, partially soluble in ethanol, ether, chloroform. When encountering oxidants, strong acids and alkalis, oxidation and decomposition reactions occur, and it can also be hydrolyzed by esterase. According to the fact that phosphatidylcholine itself contains a certain amount of oil, magnesium carbonate is used as the absorbent. Magnesium...
Embodiment 2
[0018] Embodiment 2 Preparation of valsartan and hydrochlorothiazide pharmaceutical composition tablet
[0019] Weigh respectively 20g of valsartan, 25g of hydrochlorothiazide, 150g of phosphatidylcholine, 100g of magnesium carbonate, 40g of starch, 40g of hydroxypropyl cellulose, 40g of powdered sugar, 20g of 95% ethanol, and 3.5g of silicon dioxide, according to the above method Production, a total of 1000 trial production. The preparation method of drug group I described in Example 1 was adopted to obtain drug group II.
[0020] Weigh respectively 60g of valsartan, 3g of hydrochlorothiazide, 80g of phosphatidylcholine, 100g of magnesium carbonate, 40g of starch, 40g of hydroxypropyl cellulose, 40g of powdered sugar, 20g of 95% ethanol, and 3.5g of silicon dioxide, according to the above method Production, a total of 1000 trial production. The preparation method of drug group I described in Example 1 was adopted to obtain drug group III.
Embodiment 3
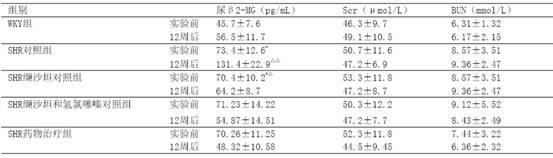
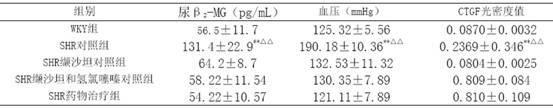
[0021] Example 3 Investigation on Kidney Protection
[0022] 40 12-week-old spontaneously hypertensive rats (SHR), 10 Wistar.Kyoto (WKY) rats of the same age, all male, were randomly divided into 3 groups, SHR control group (10 rats) SHR administration treatment group ( 10 rats) and the SHR valsartan control group (10 rats and the SHR valsartan and hydrochlorothiazide control group (10 rats), first adaptively fed for 3 days, and then the valsartan control group was given valsartan 30 mg / (kg·d ) intragastrically, SHR valsartan and hydrochlorothiazide were contrasted with a mixture / (kg d) of valsartan 22 mg and hydrochlorothiazide 8mg for intragastric administration, and the SHR drug treatment group adopted the drug group I described in Example 1 according to the content of valsartan 15 mg / (kg d), the SHR control group and the WKY group were intragastrically administered with the same amount of distilled water every day. The two kinds of rats were purchased from the Experiment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com