Primer pair and probe used for detecting AIDS treatment medicine DDI and TDF drug-resistance mutation sites and application thereof
A technology for drug resistance mutation sites and therapeutic drugs, applied in the field of biomedicine, can solve the problems of high detection cost, long time consumption, poor specificity, etc., and achieve the effects of high sensitivity, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Kits for detecting AIDS drug DDI and TDF resistance mutation sites, including:
[0058] (1) 875uL RT-PCR mixture;
[0059] RT-PCR buffer (20mM Tris-Hcl, 100mM NaCl, pH8.3) final concentration 1×;
[0060] The final concentration of dNTPs is 3mM;
[0061] MgCl 2 The final concentration is 1.6mM;
[0062] 3 pairs of primers and 3 probes for the detection of AIDS treatment drug DDI and TDF resistance mutation sites: the final concentration of the upstream and downstream primers of each ARMS primer pair is 200nM; the Taqman and kit of each ARMS primer pair The final concentration of Taqman probes for the quality control primer pair is 300nM;
[0063] Specifically, the RT-PCR mixture comprises: primer sequences SEQ ID No.1, SEQ ID No.2, SEQ ID No.4, SEQ ID No.5, SEQ ID No.6 and their respective final concentrations 0.2, 0.4, 0.2, 0.2, 0.2mmol / uL, the Taqman probe sequence is SEQ ID No.3, SEQ ID No.7, the final concentration is 0.2, 0.1mmol / uL respec...
Embodiment 2
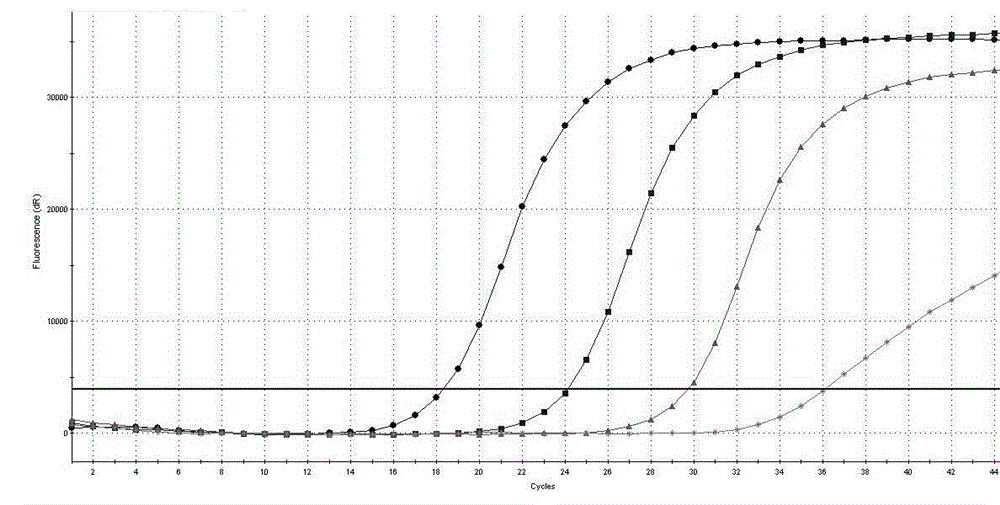
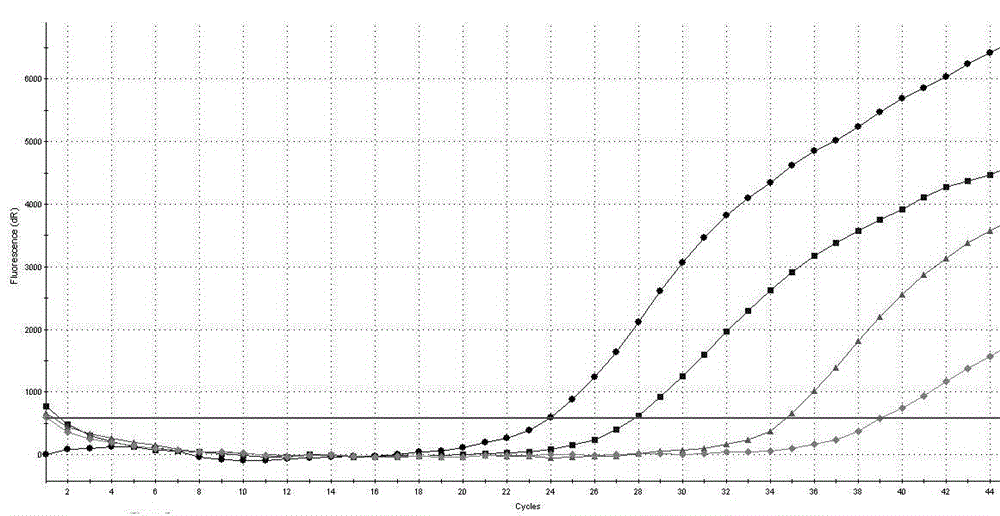
[0097] Example 2 The detection sensitivity of the AIDS treatment drug DDI and TDF drug-resistant mutation site RT-PCR detection kit of Example 1 to K65R
[0098] Prepare 17.5 μL of RT-PCR reaction solution and 2.5 μL of mixed enzyme solution for each sample, and prepare 1% of the positive control substance 1, 0.01% of the positive control substance, 0.0001% of the positive control substance, and 0.0001% of the positive control substance in Example 1. 0.000001% was diluted and used as a template for PCR reaction, and the loading volume of each diluted sample was 5uL to determine the detection sensitivity of the detection kit in the present invention to K65R (the final concentration of each component in the 20μL system was 1×RT -PCR buffer, 3 mM dNTPs, 1.6 mM MgCl2, 2U / μL M-MLV reverse transcriptase, 0.05 U / μL Taq DNA polymerase, 200nM primers, 300nM Taqman probe), the PCR reaction conditions are: 42℃ reverse transcription 10 min, one cycle; 95°C pre-denaturation for 3 minutes, ...
Embodiment 5
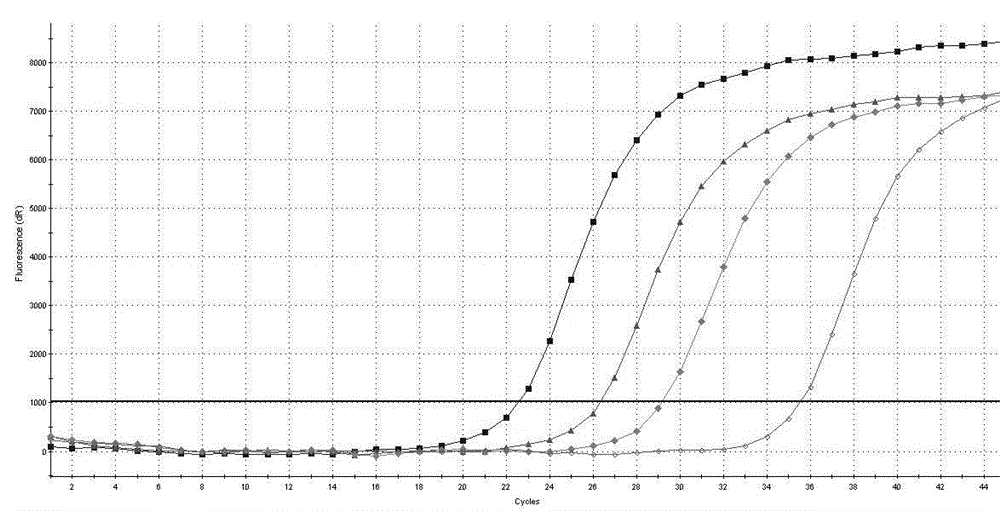
[0107] The AIDS treatment drug DDI and TDF drug-resistant mutation site RT-PCR detection kit of Example 1 is used for detection.
[0108] RT-PCR reaction solution and mixed enzyme solution were prepared according to 17.5 μL of RT-PCR reaction solution and 2.5 μL of mixed enzyme solution for each sample, and PCR amplification was performed on the positive control substance mixed solution. The PCR reaction conditions were: reverse transcription at 42°C for 10 min, one cycle; pre-denaturation at 95°C for 3 minutes, one cycle; denaturation at 95°C for 10 seconds, annealing and extension at 55°C for 40 seconds, 45 amplification cycles.
[0109] The result is as Figure 4 shown.
[0110] Depend on Figure 4 It can be seen that the Ct values of the positive controls provided in the detection kits are all below 35.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com