Method for preparing chimeric antigen receptor-T (CAR-T) cells, and prepared CAR-T cells and application thereof
A cell and mononuclear cell technology, applied in the fields of immunology and molecular biology, can solve the problems of missed patient treatment, low T cell activity, unsuitable blood collection, etc., achieving high safety, simple method, and good effectiveness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
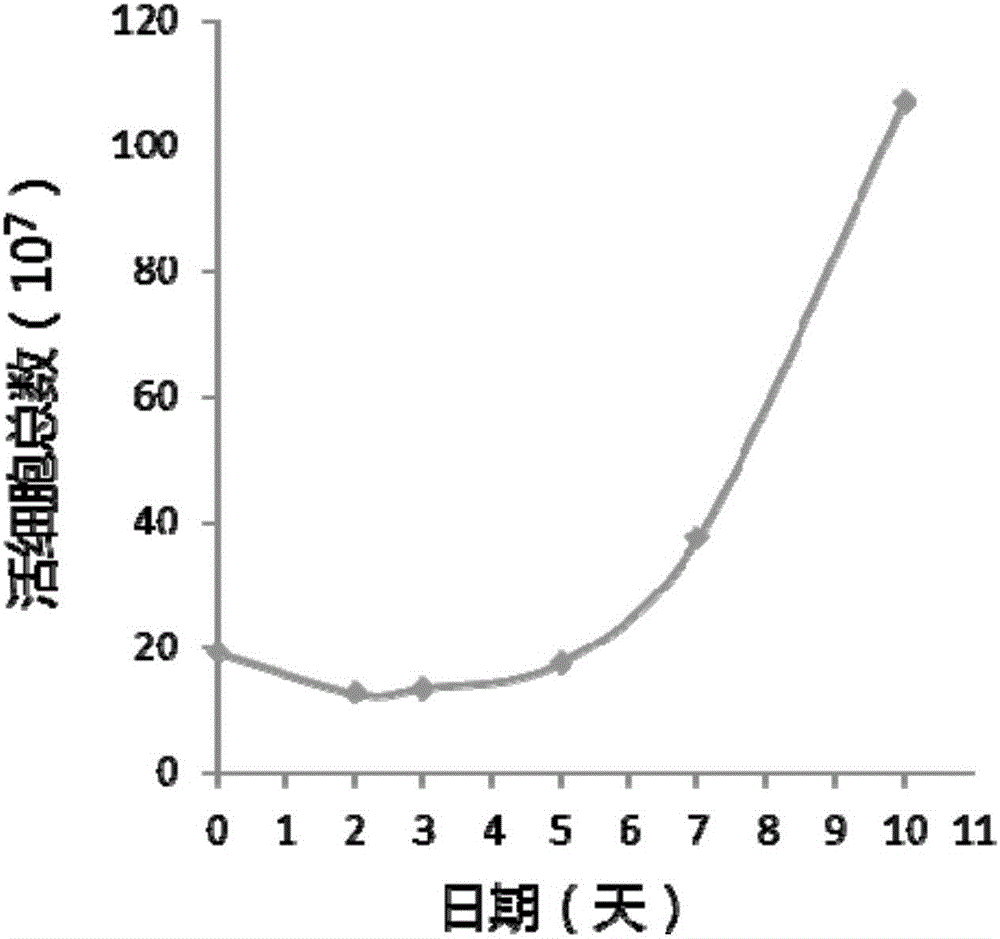
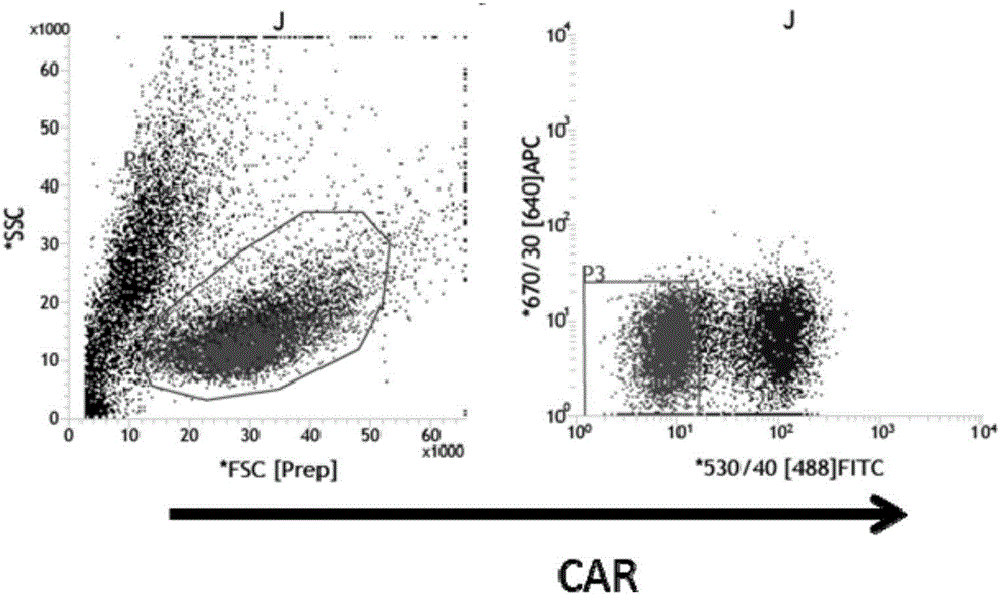
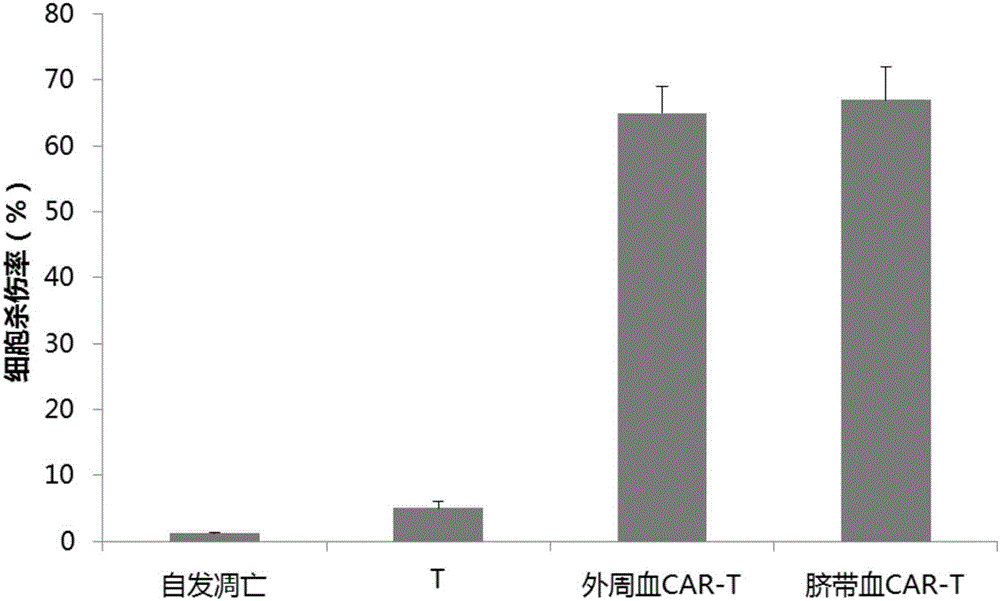
Image
Examples
Embodiment 1
[0051] Collection of cord blood
[0052] After the severed fetal umbilical cord is cleaned, blood is collected, and the wall of the blood collection tube is fully covered with anticoagulant before blood collection;
[0053] Then place the blood collection bag at a position lower than the umbilical cord, insert the blood collection needle into the lower end of the umbilical vein filling, and use gravity and extrusion to make the umbilical cord blood enter the blood collection bag;
[0054] Gently shake the blood collection bag while collecting cord blood to fully mix the cord blood and anticoagulant;
[0055] Cord blood collection can be terminated when the umbilical vein collapses, turns white, or the cord blood in the collection bag stops flowing;
[0056] The collected umbilical cord blood is temporarily stored in a refrigerator at 4°C, and transported to a GMP laboratory for separation and culture within 24 hours via a transfer car equipped with constant temperature equipm...
Embodiment 2
[0058] Preparation of umbilical cord blood mononuclear cells
[0059] Use a pipette to draw DPBS or saline into the collected or commercially purchased cord blood (volume ratio 1:1) to dilute the cord blood cells;
[0060] Slowly add the cord blood cell dilution into a centrifuge tube filled with lymphocyte separation medium (Ficoll or Histopaque-1077), centrifuge at 800g for 20-30 minutes, and absorb the buffy coat cells above the lymphocyte separation medium;
[0061] The aspirated buffy coat cells were transferred to a new centrifuge tube, and Lonza x-vivo 15 serum-free cell culture medium was added, the supernatant was discarded after centrifugation, and the cell pellet at the bottom of the centrifuge tube was kept to obtain umbilical cord blood mononuclear cells.
Embodiment 3
[0063] Enrichment of CD3 Positive T Cells from Cord Blood Mononuclear Cells
[0064] Count the umbilical cord blood mononuclear cells obtained in Example 2, add magnetic beads coupled with CD3 / CD28 antibody at a ratio of 1:1, shake gently for 20 minutes, and use a magnetic stand to obtain CD3-positive T cells .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com