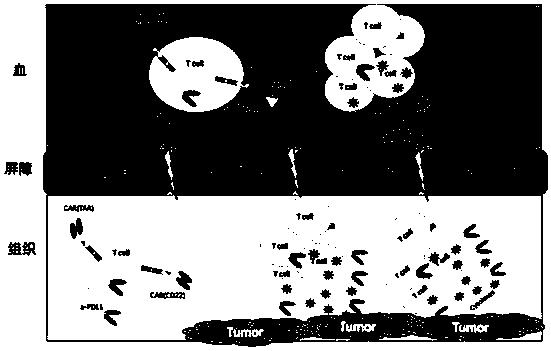
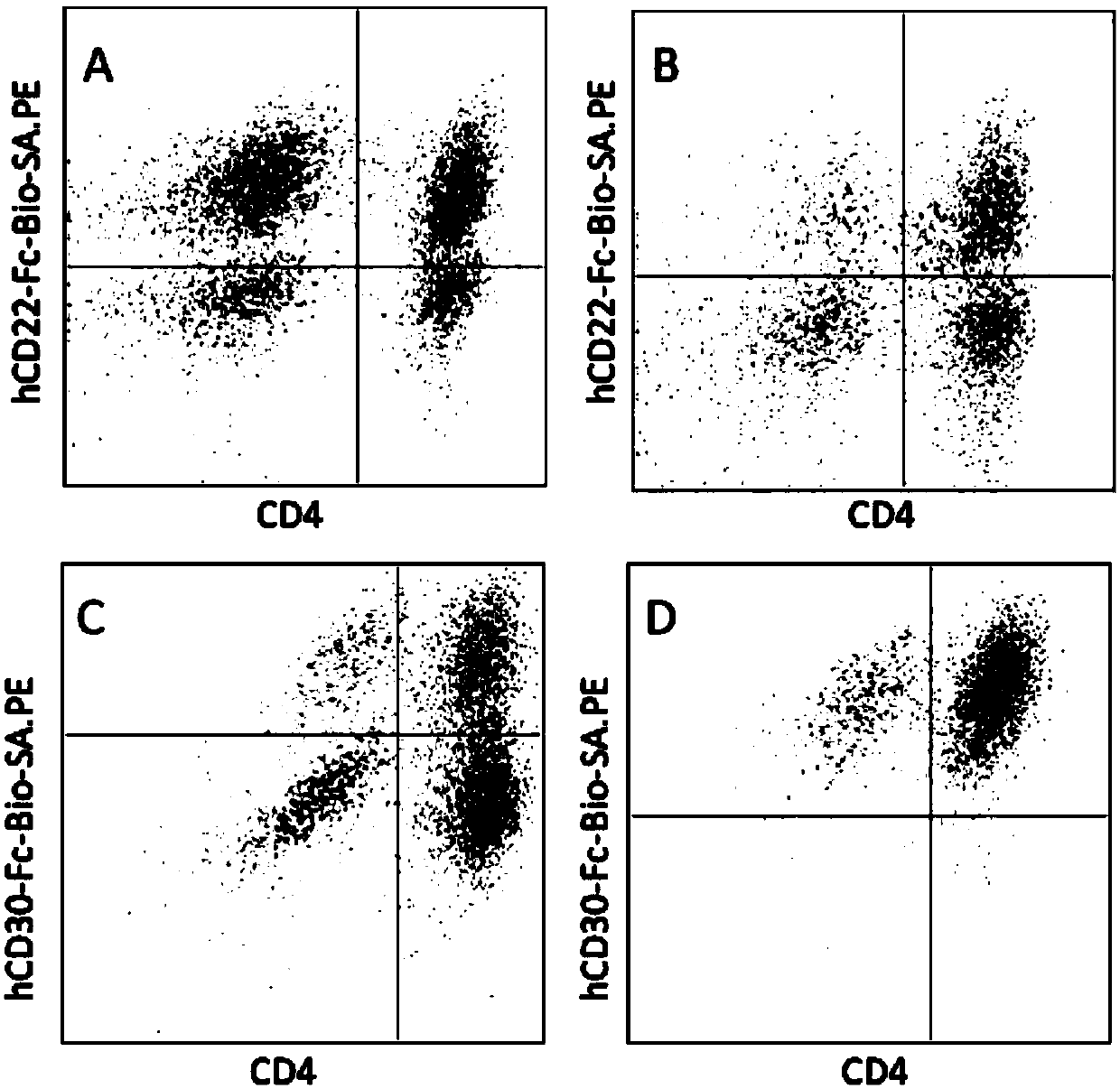
Polypeptide, nucleic acid for coding polypeptide, polypeptide-modified T lymphocyte and application of T lymphocyte
A technology of lymphocytes and B lymphocytes, applied in the biological field, can solve the problems of general curative effect, adverse reactions, high toxicity, etc., and achieve the effect of effectively treating solid tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
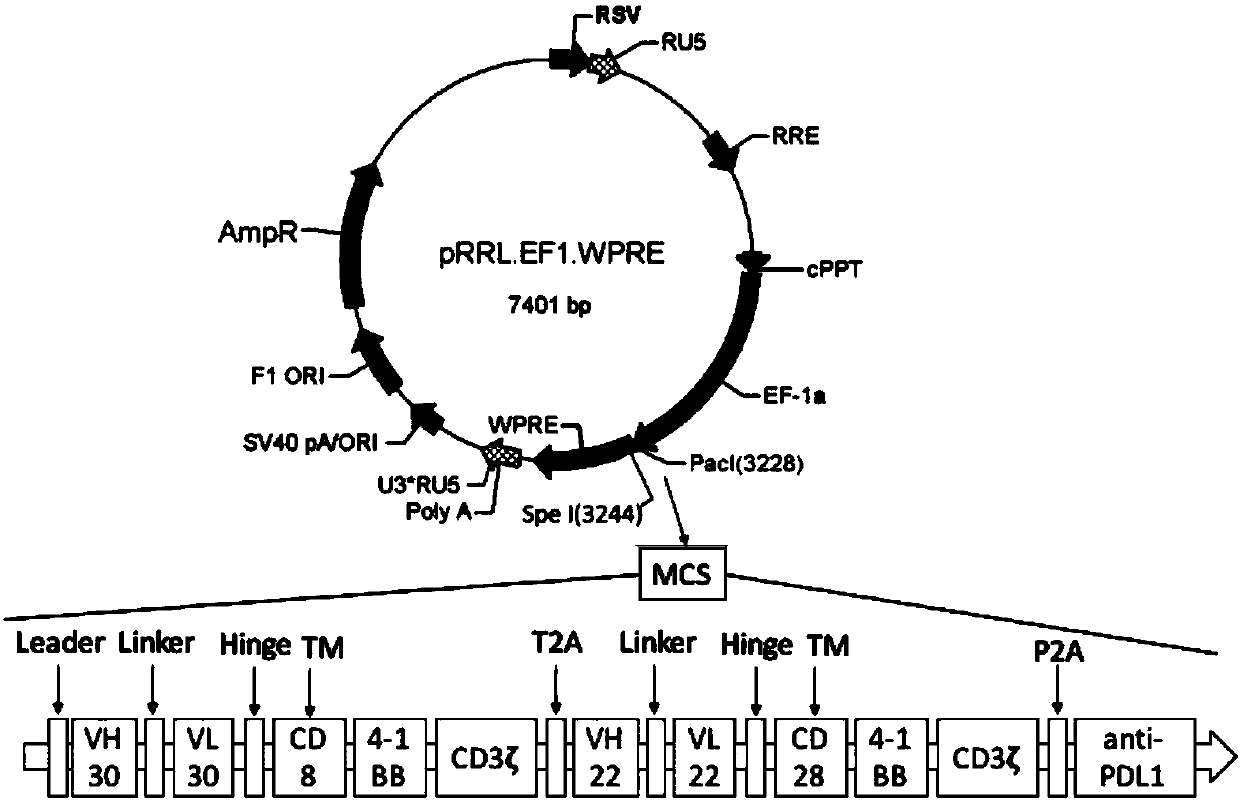
[0068] Example 1, construction of CAR(CD30)-CAR(CD22)-aPDL1 lentiviral vector
[0069] The inventors designed three target gene structures according to genetic engineering technology, and their sequences were sequentially spliced from the amino terminal to the carboxyl terminal as follows:
[0070] 1. CAR(CD30):ScFv(CD30)-Hinge(CD8)-TM(CD8)-CD137-CD3ζ
[0071] 2. CAR(CD22):ScFv(CD22)-Hinge(IgG4-short)-TM(CD28)-CD137-CD3ζ
[0072] 3. CAR(CD30)-CAR(CD22)-aPDL1:
[0073] ScFv(CD30)-Hinge(CD8)-TM(CD8)-CD137-CD3ζ-T2A-ScFv(CD22)-Hinge(IgG4-short)-TM(CD28)-CD137-CD3ζ-P2A-ScFv(PD-L1) .
[0074] Among them, ScFv(CD30)-Hinge(CD8)-TM(CD8)-CD137-CD3ζ expresses a chimeric antigen receptor targeting CD30, and its gene sequence is as follows: CD30 antibody single-chain variable region, CD8a hinge Region and transmembrane region, CD137 signal domain, CD3ζ chain intracellular region. The above sequence targets the antigen chimeric receptor sequence of CD22 in series through the self-cle...
Embodiment 2
[0081] Embodiment 2, lentiviral packaging
[0082] The operation steps of packaging are as follows:
[0083] The 293FT cell culture flask (T175) that had grown to 80%-90% was cooled from 37°C in 5% CO 2 Take it out from the cell culture incubator, add 2 mL of EDTA-free-0.25% Trypsin to digest, collect and wash the cells, add 4.5×10 per 10 cm cell culture dish 6 For each cell, add 9mL DMEM medium, shake gently, put in 37℃5%CO 2 cultured in an incubator.
[0084] On day 2, 500 μL per plate Buffer, 6 μg target gene, 3 μg psPAX2, 1.5 μg pMD2.G, mix the above solutions evenly. Add to the mixture 25μL / 10cm plate, mix well again, and let stand at room temperature for 10min. Warm the 293FT cells used for packaging virus from 37°C 5% CO 2 Take it out of the cell culture incubator, add the above mixture evenly to each plate, shake gently, and put it in 37°C 5% CO 2 in the incubator. After 4 hours, discard the old medium, add 10mL of preheated PBS to wash the cells, then add 9...
Embodiment 3
[0086] Example 3, preparation of CAR-T cells
[0087] 1. Cell expansion (Day0)
[0088] Aseptically collect 50-100ml of venous blood from the patient, and perform density gradient centrifugation on the blood sample to obtain peripheral blood mononuclear cells (PBMC). CD3+ cells were sorted with CD3 sorting magnetic beads (Miltenyi, Germany). The sorted positive cells were first resuspended with 2ml of medium, and T cell activation magnetic beads (Gibco, USA) were added according to the ratio of positive cells to magnetic beads at 1:1. Then spread a 24-well plate according to 3×10^5 cells / 500 μl medium / well, and move to 37°C, 5% CO 2 The cells were cultured in an incubator for 2 days.
[0089] 2. Cell transfection (Day2)
[0090] The 24-well plate was taken out of the incubator in advance and placed in a biological safety cabinet, and allowed to cool to room temperature. Then add Protamine Sulfate according to 1‰ (V / V). After the virus was melted, it was added to the 24-w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com