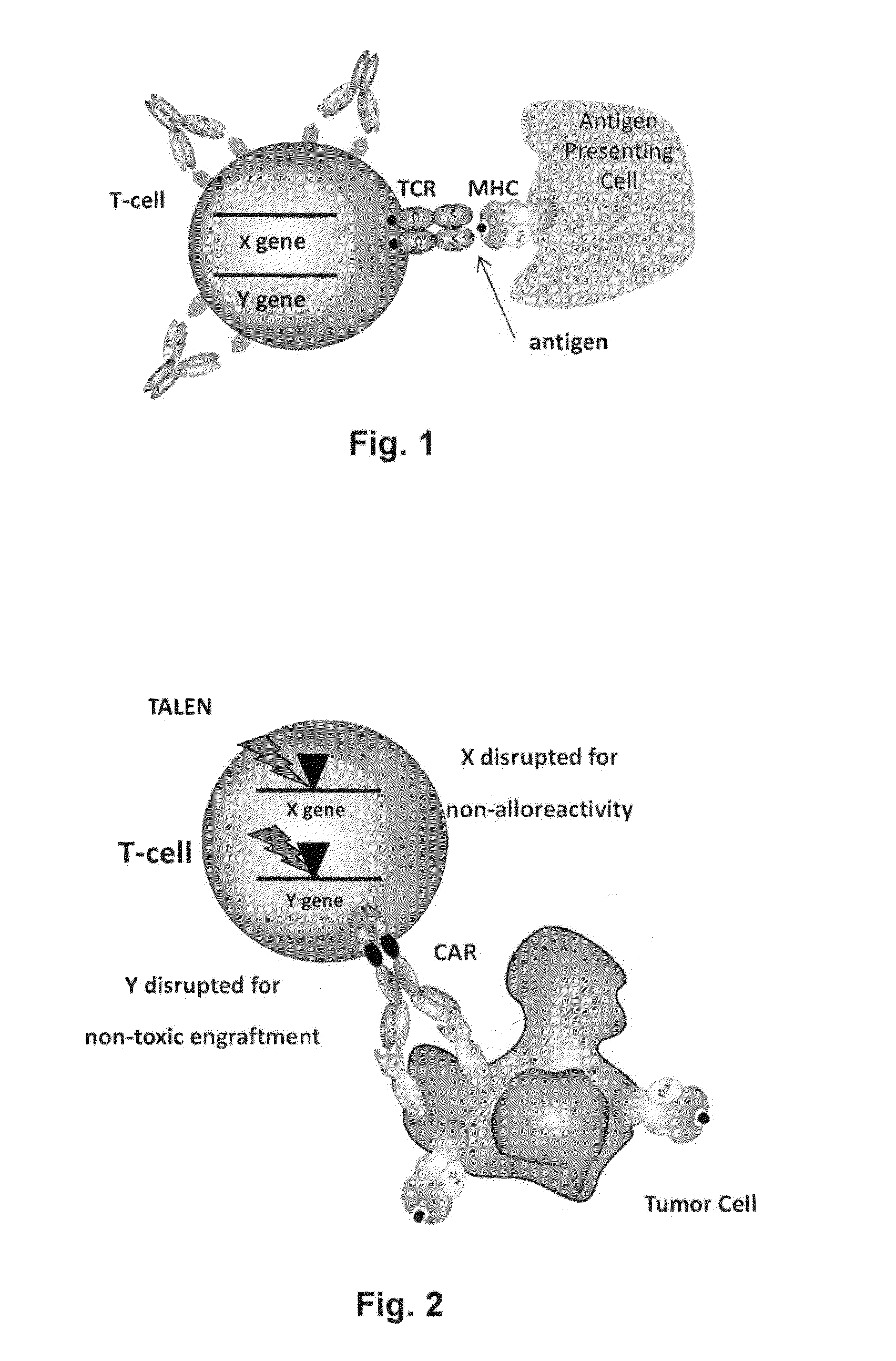
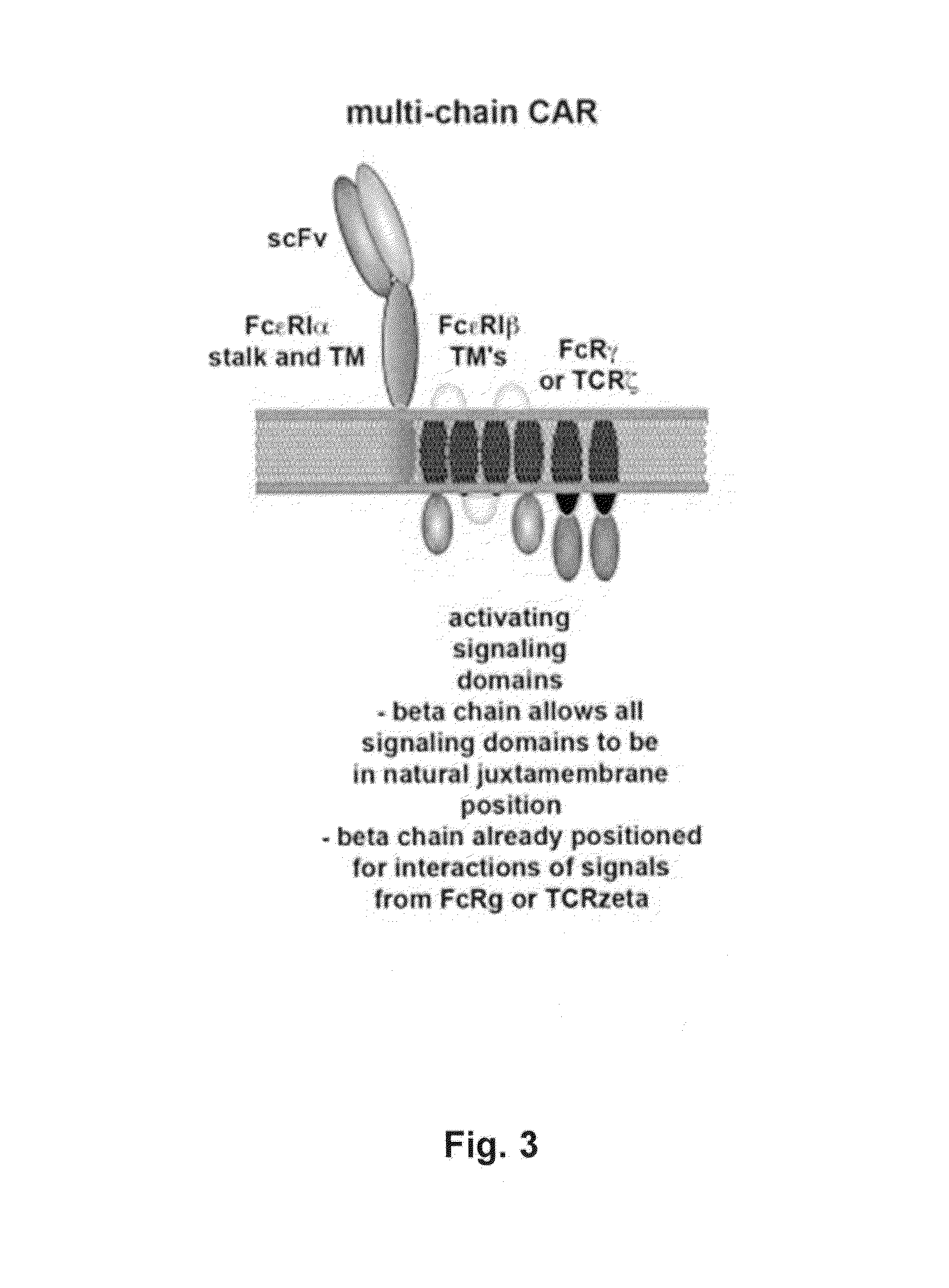
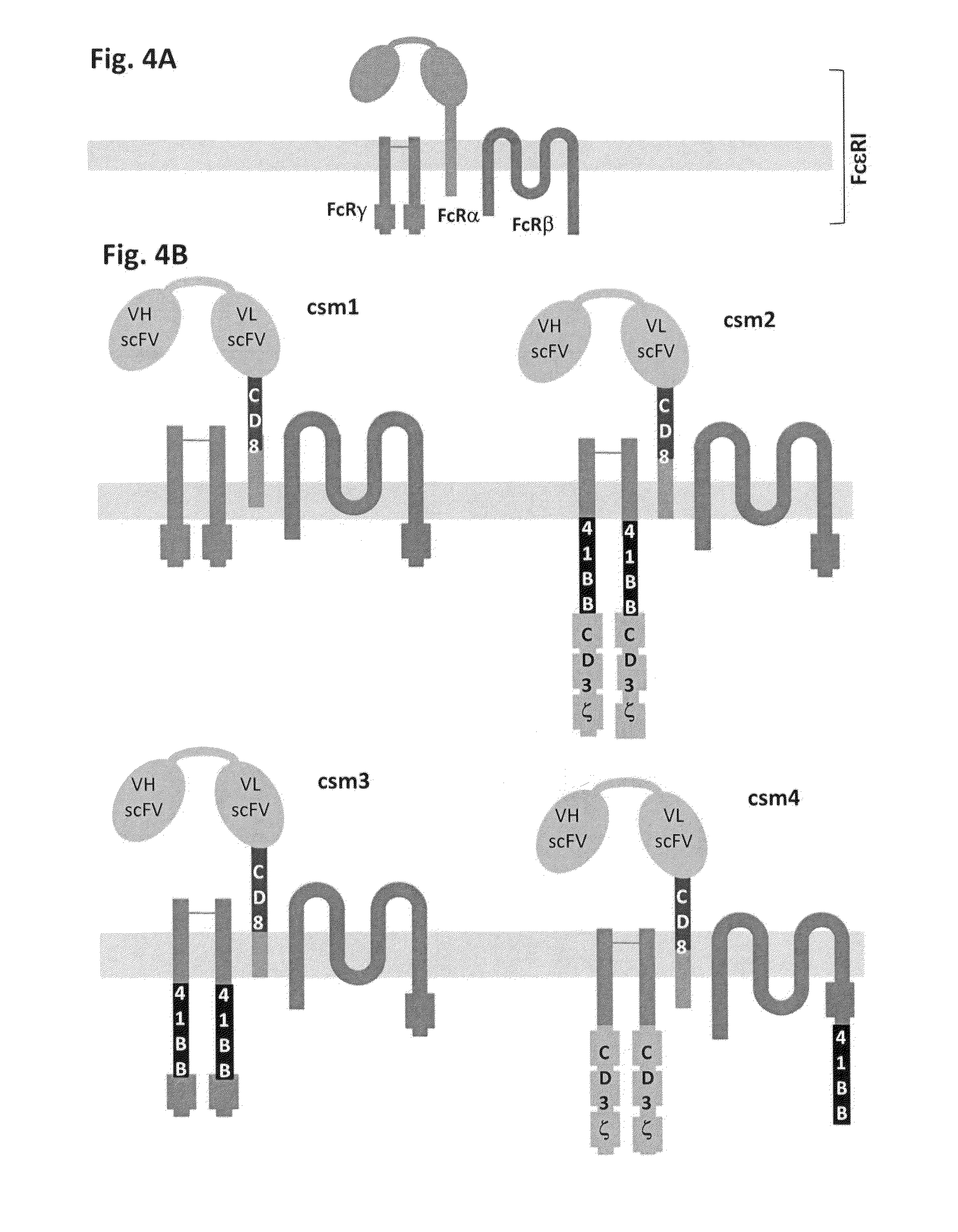
Methods for engineering allogeneic and highly active t cell for immunotherapy
a technology of immunotherapy and t cells, applied in the direction of peptide/protein ingredients, cell culture active agents, fusion polypeptides, etc., can solve problems such as interfering with their function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
TALE-Nucleases Cleaving the Human GR Gene
[0296]6 heterodimeric TALE-nucleases targeting exons of the human GR gene were designed and produced. Table 1 below indicates the target sequences cleaved by each TALE-nuclease. GR TALE-nuclease was composed of two independent entities (called half TALE-nucleases) each containing a repeat sequence engineered to bind and cleave GR target sequences consisting of two 17-bp long sequences (called half targets) separated by a 15-bp spacer.
TABLE 1Description of the GR TALE-nucleases and sequences of the TALE-nucleases targetsites in the human GR gene.Half TALE-nucleaseTarget nameTarget sequenceRepeat sequencesequenceGRex2TATTCACTGATGGACTCRepeat GRex2-LPT9-L1GRex2-L TALENcaaagaatcattaac(SEQ ID NO: 7)(SEQ ID NO: 19)TCCTGGTAGAGAAGAAARepeat-GRex2-LPT9-R1GRex2-R TALEN(SEQ ID NO: 1)(SEQ ID NO: 8)(SEQ ID NO: 20)GRex3T2TGCCTGGTGTGCTCTGARepeat-GRex3T2-L1GRex3T2-L TALENtgaagcttcaggatg(SEQ ID NO: 9)(SEQ ID NO: 21)TCATTATGGAGTCTTAARepeat-GRex3T2-R1GRex3T2-R TA...
example 2
TALE-Nucleases Cleaving the Human CD52 Gene, the Human T-Cell Receptor Alpha Constant Chain (TRAC) and the Human T-Cell Receptor Beta Constant Chains 1 and 2 (TRBC)
[0308]As described in example 1, heterodimeric TALE-nucleases targeting respectively CD52, TRAC and TRBC genes were designed and produced. The targeted genomic sequences consist of two 17-bp long sequences (called half targets) separated by an 11 or 15-bp spacer. Each half-target is recognized by repeats of half TALE-nucleases listed in table 5. The human genome contains two functional T-cell receptor beta chains (TRBC1 and TRBC2). During the development of alpha / beta T lymphocytes, one of these two constant chains is selected in each cell to be spliced to the variable region of TCR-beta and form a functional full length beta chain. The 2 TRBC targets were chosen in sequences conserved between TRBC1 and TRBC2 so that the corresponding TALE-nuclease would cleave both TRBC1 and TRBC2 at the same time.
TABLE 5Description of t...
example 3
TALE-Nucleases Cleaving the Human CTLA4 Gene and the Human PDCD1 Gene
[0317]As described in example 1, heterodimeric TALE-nucleases targeting respectively PDCD1 and CTLA4 genes were designed and produced. The targeted genomic sequences consist of two 17-bp long sequences (called half targets) separated by an 11 or 15-bp spacer. Each half-target is recognized by repeats of half TALE-nucleases listed in table 10.
TABLE 10Description of the CTLA4 and PDCD1 TALE-nucleases and sequences of the TALE-nucleases target sites in the human corresponding genes.TargetTarget sequenceRepeat sequenceHalf TALE-nucleaseCTLA4_T01TGGCCCTGCACTCTCCTRepeat CTLA4_T01-LCTLA4_T01-L TALENgttttttcttctctt(SEQ ID NO: 79)(SEQ ID NO: 89)CATCCCTGTCTTCTGCARepeat CTLA4_T01-RCTLA4_T01-R TALEN(SEQ ID NO: 74)(SEQ ID NO: 80)(SEQ ID NO: 90)CTLA4_T03TTTTCCATGCTAGCAATRepeat CTLA4_T03-LCTLA4_T03-L TALENgcacgtggcccagcc(SEQ ID NO: 81)(SEQ ID NO: 91)TGCTGTGGTACTGGCCARepeat CTLA4_T03-RCTLA4_T03-R TALEN(SEQ ID NO: 75)(SEQ ID NO: 82...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cytotoxicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com