Checkpoint Blockade and Microsatellite Instability
a checkpoint blockade and microsatellite technology, applied in the field of cancer, can solve the problem of accumulation of sequencing errors in microsatellites, microsatellite instability (msi) is the accumulation of sequencing errors, and achieve the effect of high mutational burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MSI Testing
[0041]MSI testing is already standardized and performed in CLIA-certified laboratories without need for assay development. Archived tumor samples or newly obtained biopsies will be used for determining MSI. MSI status will be performed locally by CLIA certified immunohistochemistry (IHC) or PCR based tests for eligibility. Evaluable patients will be confirmed using the MSI Analysis System from Promega at Johns Hopkins. This test will determine MSI status through the insertion or deletion of repeating units in the five nearly monomorphic mononucleotide repeat markers (BAT-25, BAT-26, MONO-27, NR-21 and NR-24). At least 2 MSI loci are required to be evaluable in Cohorts A and C. Patients may be assigned to a new cohort and / or replaced based on the Promega test results.
example 2
Methods
[0042]Patients
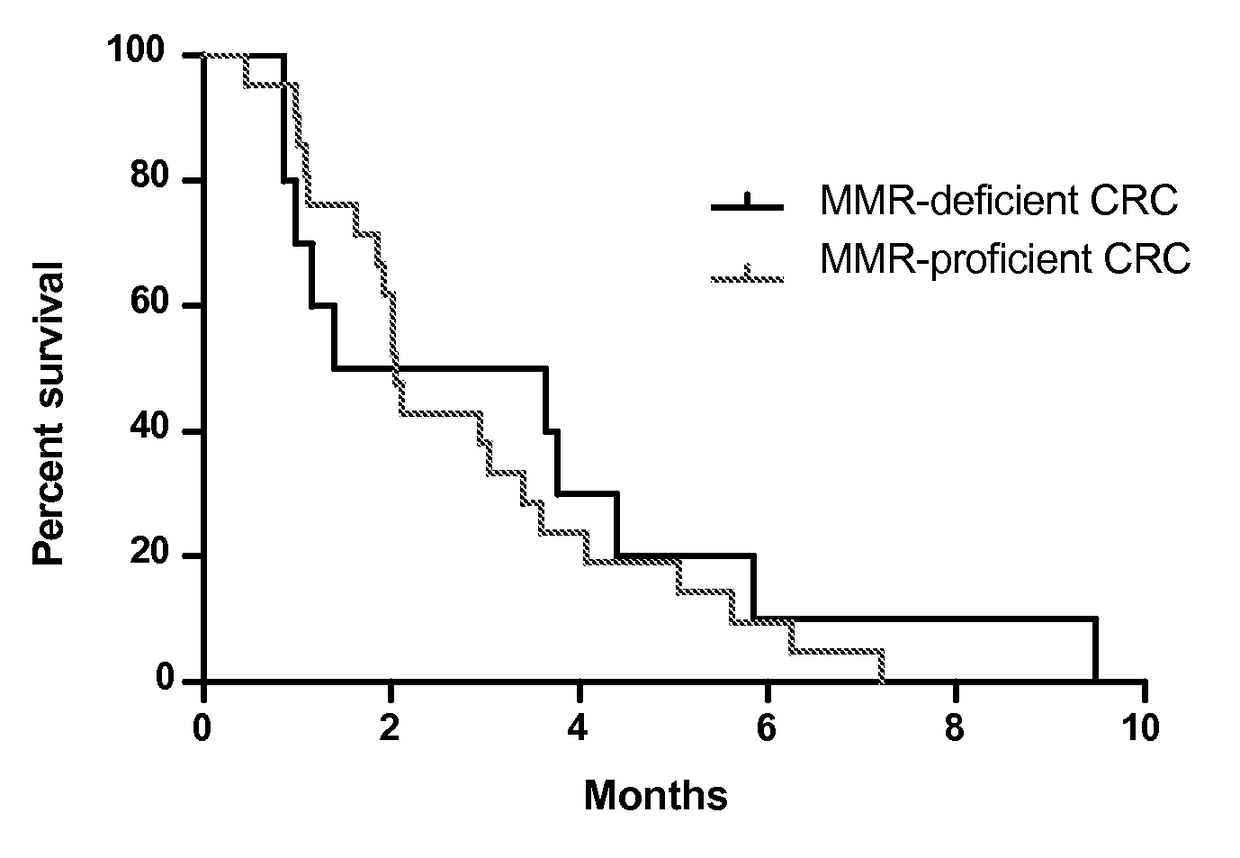
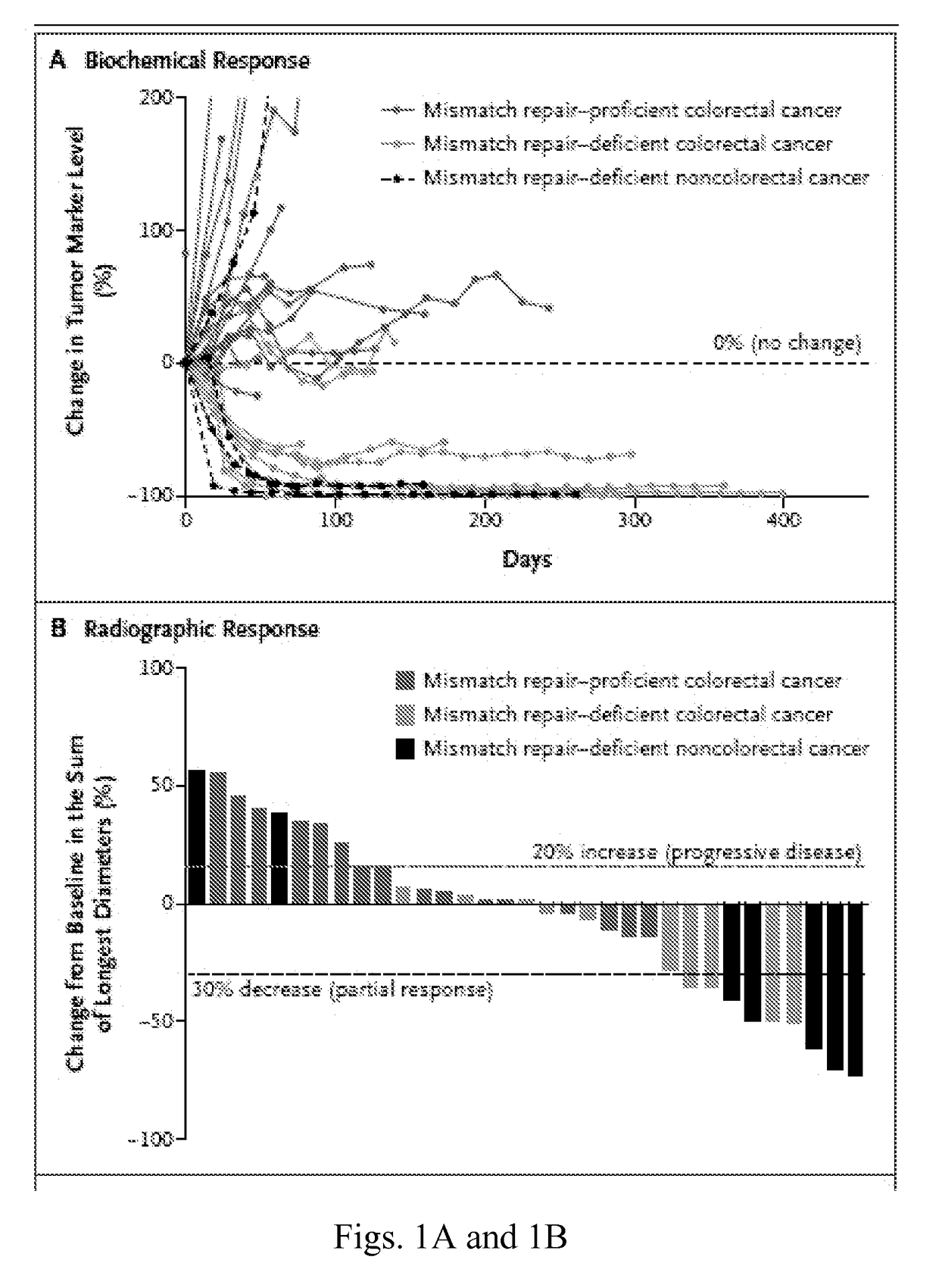
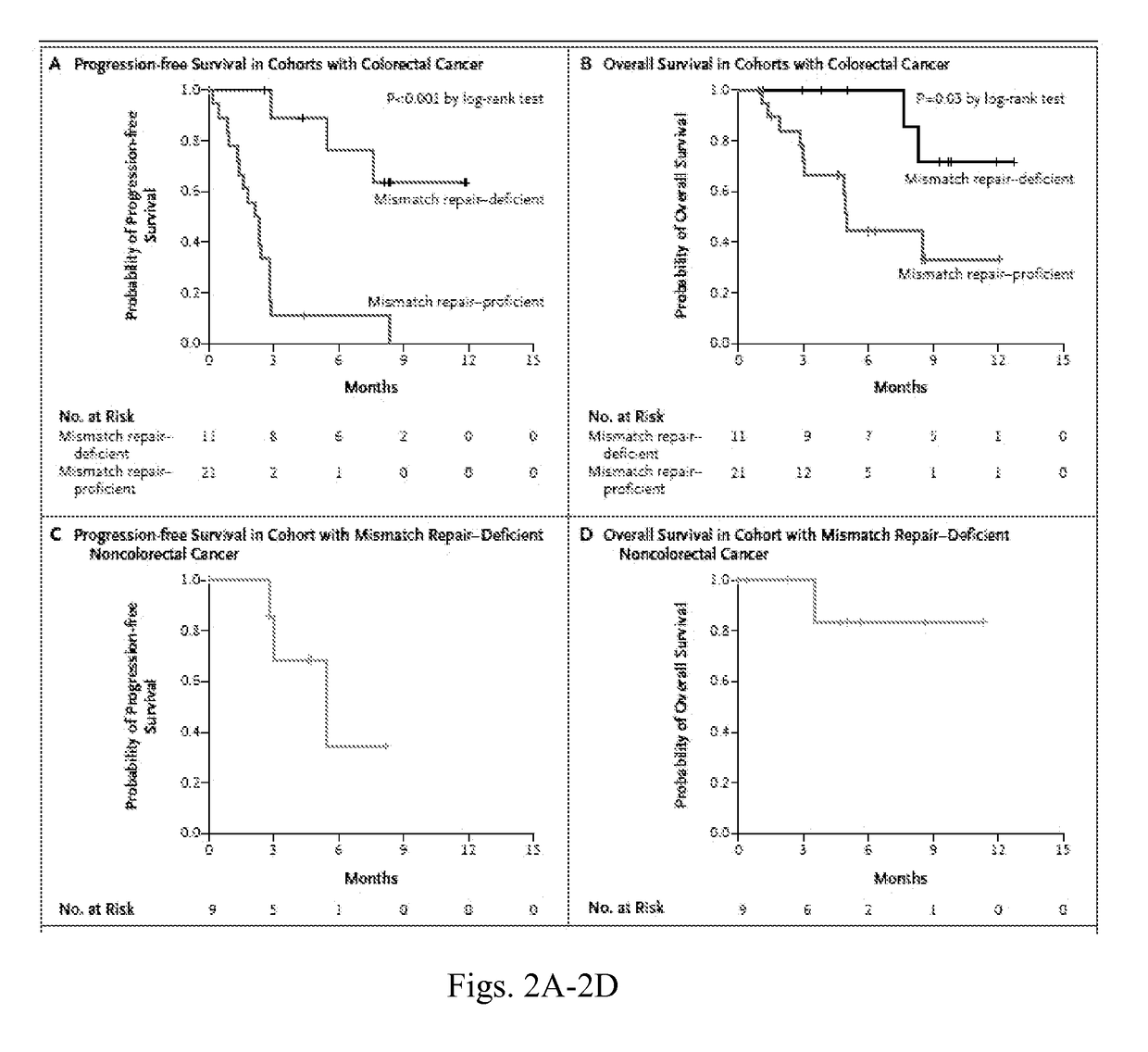
[0043]Treatment-refractory progressive metastatic cancer patients for this phase 2 study were recruited from three participating centers (Table 1). Three cohorts were evaluated: Cohort A was composed of patients with MMR-deficient colorectal adenocarcinomas; Cohort B was composed of patients with MMR-proficient colorectal adenocarcinomas; and Cohort C was composed of patients with MMR-deficient cancers of types other than colorectal.
[0044]Study Oversight
[0045]The protocol, which can be found at NEJM.org, was approved by each site's institutional review boards, and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All the patients provided written informed consent before study entry. The principal investigator (D.L.) and study sponsor (L.A.D.) were responsible for oversight of the study. Merck donated the study drug, reviewed the final drafts of the proto...
example 3
Supplementary Methods
[0055]Patients
[0056]To be eligible for participation in this study, patients had to be at least 18 years of age, have histologically confirmed evidence of previously-treated, progressive carcinoma. All patients underwent MMR status testing prior to enrollment. All patients had at least one measurable lesion as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1, and adequate hematologic, hepatic, and renal function. Eligible patients with CRC must have received at least 2 prior cancer therapies and patients with other cancer types must have received at least 1 prior cancer therapy. Patients with untreated brain metastases, history of HIV, hepatitis B, hepatitis C, clinically significant ascites / effusions, or autoimmune disease were excluded.
[0057]Study Oversight
[0058]Initial drafts of the manuscript were prepared by a subset of the authors and all autho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com