Affinity peptide L8 of PD-1 (programmed death-1) protein extracellular domain and application thereof
A PD-1, extracellular segment technology, applied to affinity peptide L8 and its application field, can solve problems such as poor curative effect, achieve good practical application and promotion significance, improve survival time, and obvious effects of inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In order to facilitate those skilled in the art to implement the present invention, the inventor briefly describes the screening process of the affinity peptide L8 of the extracellular portion of the PD-1 protein as follows:
[0025] 1. The expression and purification of PD-1 extracellular protein, the brief steps are as follows:
[0026] (1) First construct the pET-28a(+)-hPD-1 recombinant plasmid containing the extracellular sequence of PD-1;
[0027] (2) Transform the recombinant plasmid into Escherichia coli Transetta (DE3);
[0028] (3) IPTG induces the expression of the target protein;
[0029] (4) Purify the protein with a nickel metal chelate affinity chromatography column, and obtain the active target protein after dialysis and refolding.
[0030] Specific steps are as follows:
[0031] (1) Using PHA (phytohemagglutinin)-stimulated healthy human PBMCs (peripheral blood mononuclear cells) as materials, total RNA was extracted using Trizol kit, and its cDNA...
Embodiment 2
[0057] According to the L8 peptide screened in Example 1, the affinity peptide L8 was artificially synthesized.
[0058] The affinity peptide L8 of the extracellular segment of PD-1 protein is synthesized by Fomc solid-phase peptide synthesis method, and the brief steps are as follows:
[0059] (1) Select Rink resin to covalently connect the first Fmoc-amino acid carboxyl group of the C-terminal of the peptide to be synthesized, that is, PD-1 affinity peptide L8, and then use the N-terminal of the amino acid as the starting point for the synthesis of the polypeptide. And let it undergo a dehydration condensation reaction with the carboxyl terminal of the next amino acid to form a peptide bond;
[0060] (2) Then, deprotect the protecting group of the Fmoc-amino acid at the N-terminus, and then react the N-terminus of the second amino acid with the carboxyl group of the following amino acid, and repeat this process until the synthesis of the peptide is completed;
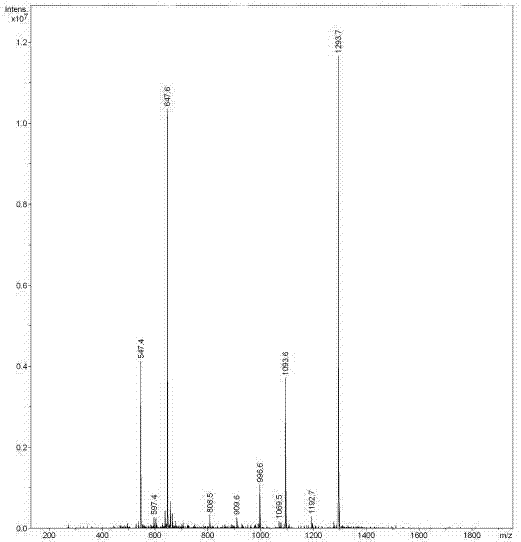
[0061] (3) F...
Embodiment 3
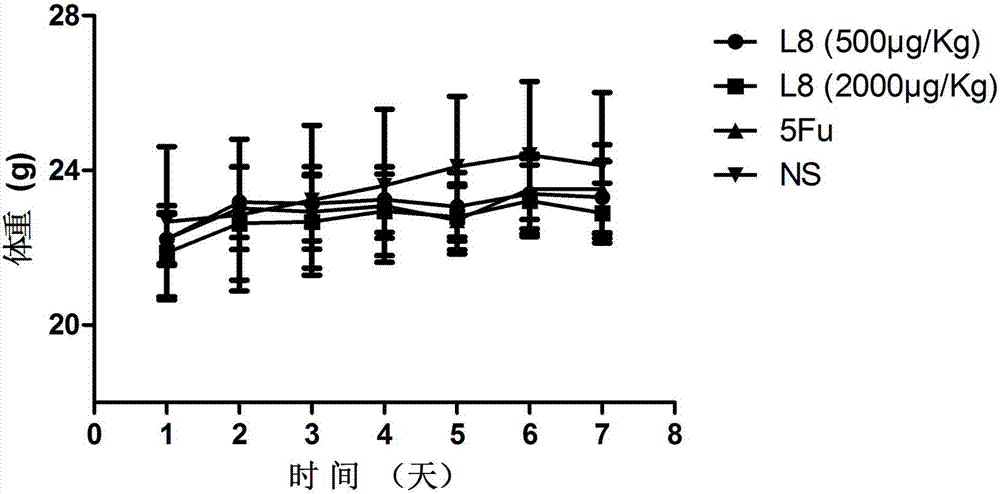
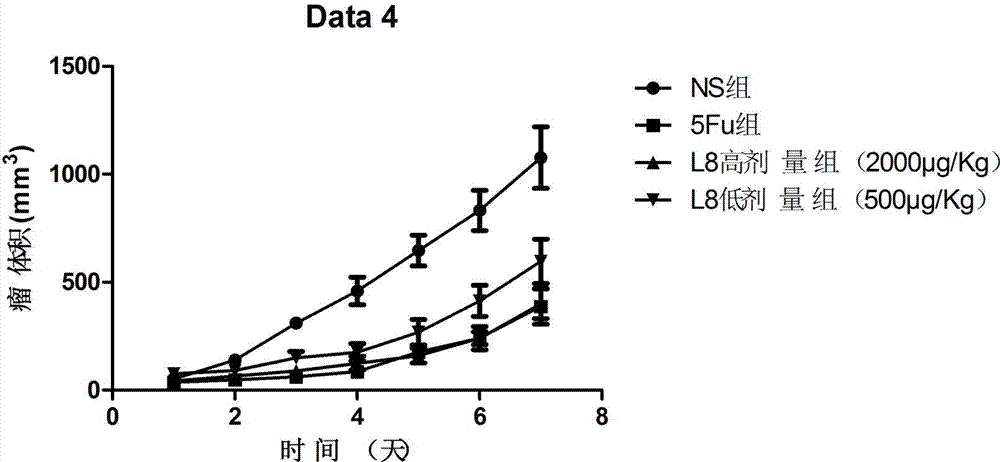
[0085] In order to further test the anti-tumor activity of the affinity peptide L8, the inventor further carried out anti-tumor related experiments with the affinity peptide L8 prepared in Example 2. The specific experimental conditions are as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com