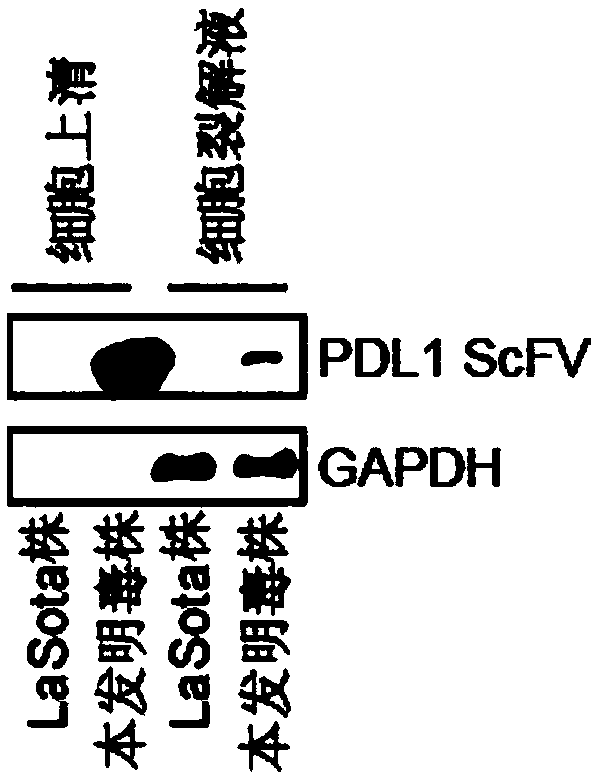
Preparation method and application of newcastle disease oncolytic virus expressing PD-L1 single chain antibody
A Newcastle disease virus, PD-L1 technology, applied in the field of Newcastle disease oncolytic virus, can solve problems such as reduced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
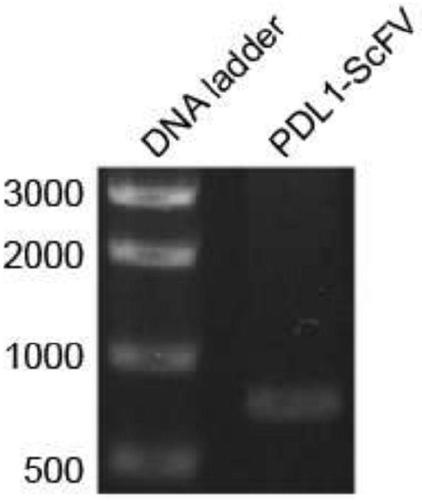
Image
Examples
Embodiment
[0026] 1) Preparation of pBRN-FL (112-RRQRRF-117)
[0027] By mutation PCR, the amino acid residues 112-117 of the F protein of pBRN-FL full-length clone were mutated to 112-R-R-Q-R-R-F-117: using pBRN-FL as a template, PCR was performed with the primers in Table 1, and the reaction system was:
[0028]
[0029] ddH 2 O make up to 20ul
[0030] The reaction program is: ①98°C, 5min; ②98°C, 20sec; ③68°C, 90sec; ④Steps 2-3 cycled 18 times; ⑤68°C, 15min;
[0031] After the reaction is completed, take 9ul of the reaction product, add 1ul of DNA restriction endonuclease DpnI, and react at 37°C for 2h; after the reaction, take 100ul of competent DH5αE. Then heat shock at 42°C for 90sec; then ice bath for 2min, then add 500ul antibiotic-free LB medium, recover in a 37°C constant temperature shaking incubator at 180rpm for 30min; apply to LB solid medium plate containing ampicillin antibiotic, cultivate overnight in an incubator at 37°C, pick Take a single clone to 5ml containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com