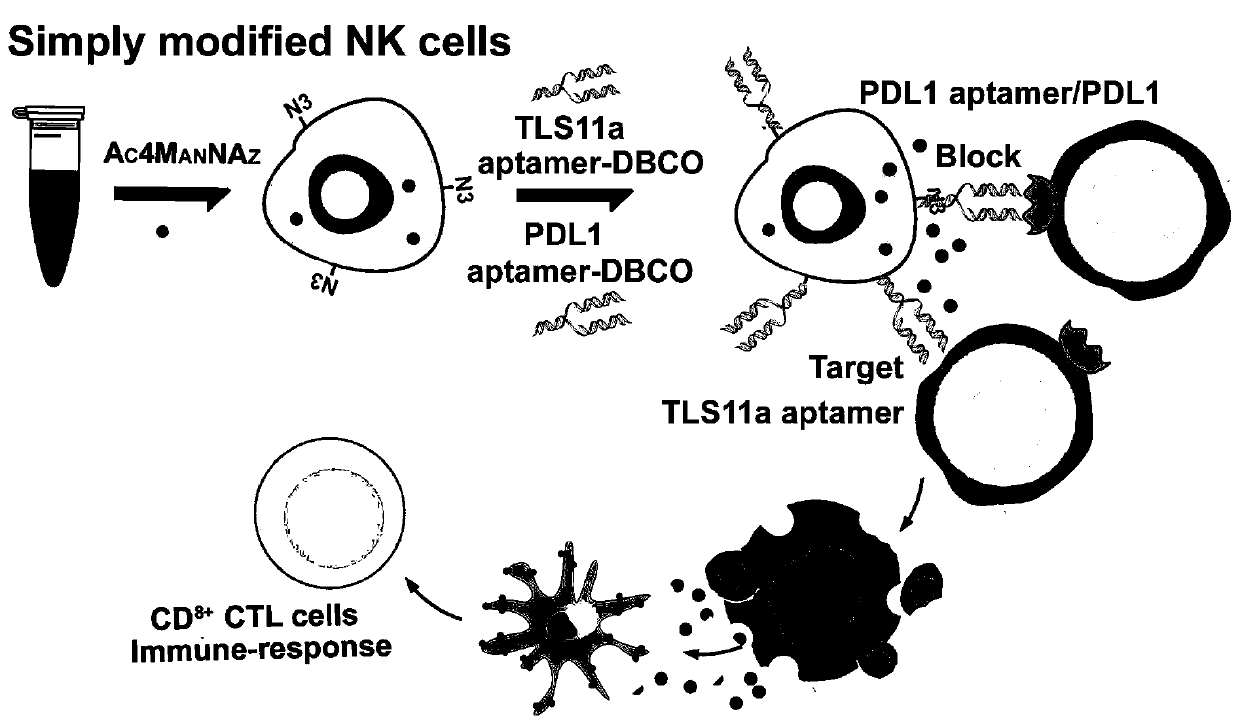
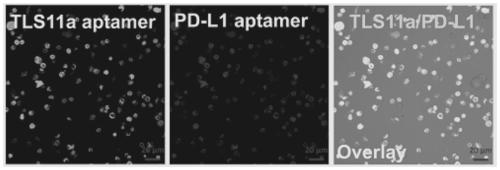
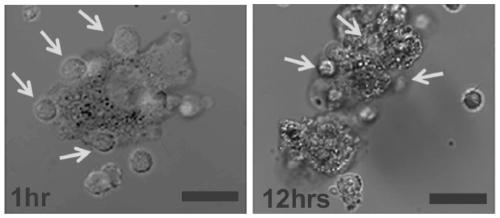
Artificial modified natural killer cell and preparation and application thereof
A lethal and natural technology, applied in animal cells, vertebrate cells, cell culture active agents, etc., can solve problems such as poor in vivo stability, achieve good biocompatibility, improve targeting nucleic acid titer, and improve active target. The effect of the ability to kill
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Take 30 mL of EDTA anticoagulated whole blood, add 30 mL of normal saline to dilute. Take a 50ml EP tube, add 20ml of Ficoll separation solution, slowly add whole blood diluent without destroying the liquid layer, and then centrifuge at 0 under the condition that the centrifuge rise and fall speed is 0, centrifuge at 800G for 20min; collect the cloudy white blood cell layer in the middle , washed twice with normal saline, centrifuged at 600G for 5 min, collected the precipitate and counted.
[0047] PBMC cells (total number of cells 3 × 10 7 A) were resuspended in 30 mL of KBM581 medium, added gentamicin 80IU / mL, recombinant human IL2 (Jiangsu Kingsley) 200IU / mL, autologous plasma 5%, added irradiated engineered IL-21NK cells ( Hangzhou Zhong Ying, 3×10 8 ), placed at 37°C, 5% CO 2 Cultivated in an incubator. From the 3rd day of culture, supplement KBM581 medium (the volume of the medium added each time does not exceed twice the existing volume) and cytokines (genta...
Embodiment 2
[0050] The artificially engineered natural killer (NK) cells prepared in Example 1 can be used for adoptive immunotherapy of liver cancer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com