Fusion protein for restoring functions of exhaustion immune cells and application thereof
A technology of immune cells and fusion proteins, applied in the field of fusion proteins, can solve problems such as specific cells that cannot be targeted for exhaustion, achieve good clinical prospects, enhance the effect of inhibiting tumor growth and restoring functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
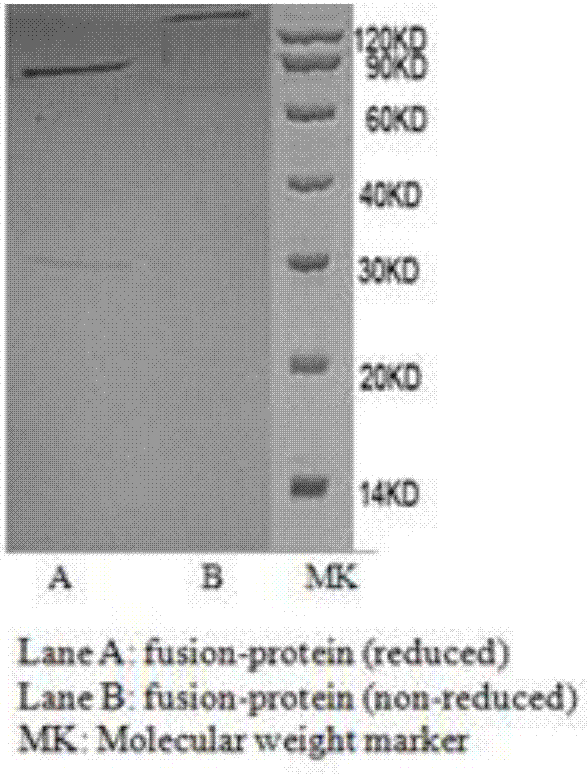
[0043] This example is the gene construction, production and purification of recombinant fusion protein.
[0044] According to the C-terminal of human PD-1 antibody heavy chain (SEQ ID NO: 6) and the N-terminal amino acid sequence of interleukin 2 (SEQ ID NO: 14), through gene synthesis, restriction digestion and further cloning, the two parts were passed through 17 non- Functional amino acids (SGGGGSGGGGSGGGGSG) are connected to form the fusion protein heavy chain construction gene (SEQ ID NO: 23), and then transferred into the eukaryotic animal expression vector pcDNA3.1. Through gene synthesis, restriction digestion and further cloning, the light chain (SEQ ID NO: 7) gene of the PD-1 antibody was transferred into the eukaryotic expression vector pcDNA3.1. Finally, the heavy chain expression vector and light chain expression vector of the fusion protein were simultaneously transfected into Chinese hamster ovary cells (CHO). Place the transfected cells at 37℃, 5% CO 2 After cul...
Embodiment 2
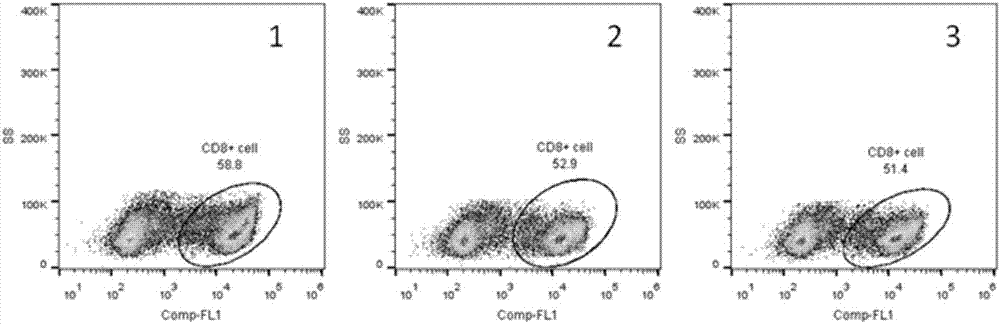
[0046] This example is the determination of the activity and function of the bifunctional recombinant fusion protein on human PBMC cells cultured in vitro.
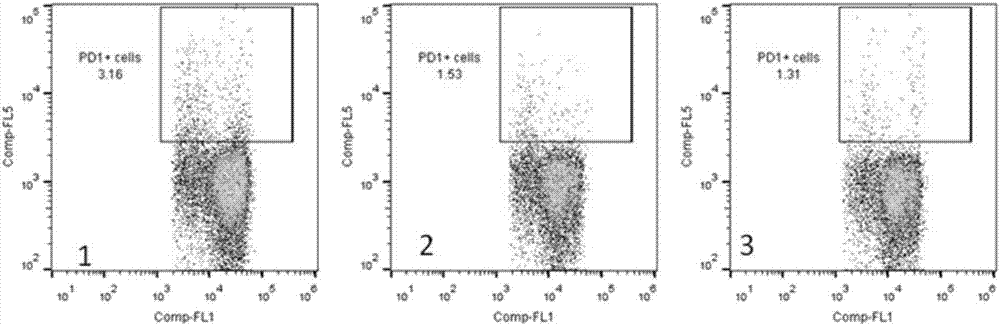
[0047] Human peripheral blood was separated and purified by lymphocyte density gradient centrifugation (Ficoll). The cell density was diluted to 5×10 in X-Vivo15 medium in a 24-well plate. 6 / ml, add test protein to a final concentration of 2μg / ml. Incubate at 37°C for 30 minutes. After centrifugation, change the X-Vivo15 medium to the final cell density of 5x10 5 / ml. Then put it at 37℃, 5% CO 2 After culturing in the incubator for 72 hours, they were taken out. After the cells were collected, they were stained with CD8 and PD-1 antibodies, and phenotype determination and data analysis were performed by flow cytometry. figure 2 Shows that the unfused PD-1 antibody ( figure 2 Part 2) and Interleukin 2 ( figure 2 Part 3) compared to fusion protein treatment ( figure 2 Part 1) can significantly increase the proportion of CD...
Embodiment 3
[0049] This example shows the effect of bifunctional recombinant fusion protein on effector cells in vivo.
[0050] Small black mice were injected with fusion protein (4μg / mouse / day) or interleukin-2 (40μg / mouse / day) or PBS (control group) through the abdominal cavity for 3 consecutive days. On the 4th day, peripheral blood was taken from the tail and used anti-mouse CD8 And NK1.1 flow cytometry antibody staining and testing, data analysis such as Figure 4 . With the control group ( Figure 4 Part 1, 4.0%) and Interleukin 2 group ( Figure 4 Compared with part 2, 15.3%), the recombinant fusion protein significantly increased the proportion of NK cells in lymphocytes ( Figure 4 Part 3, 27.0%). At the same time, in the CD8+ T cell part, and the control group ( Figure 4 Part 1, 15.5%) Compared to native interleukin 2 did not increase the proportion of CD8+ cells ( Figure 4 Part 2, 15.1%), and the proportion of CD8+ T cells in peripheral blood of mice treated with fusion pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com