Patents
Literature
46 results about "Flaviviridae Infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
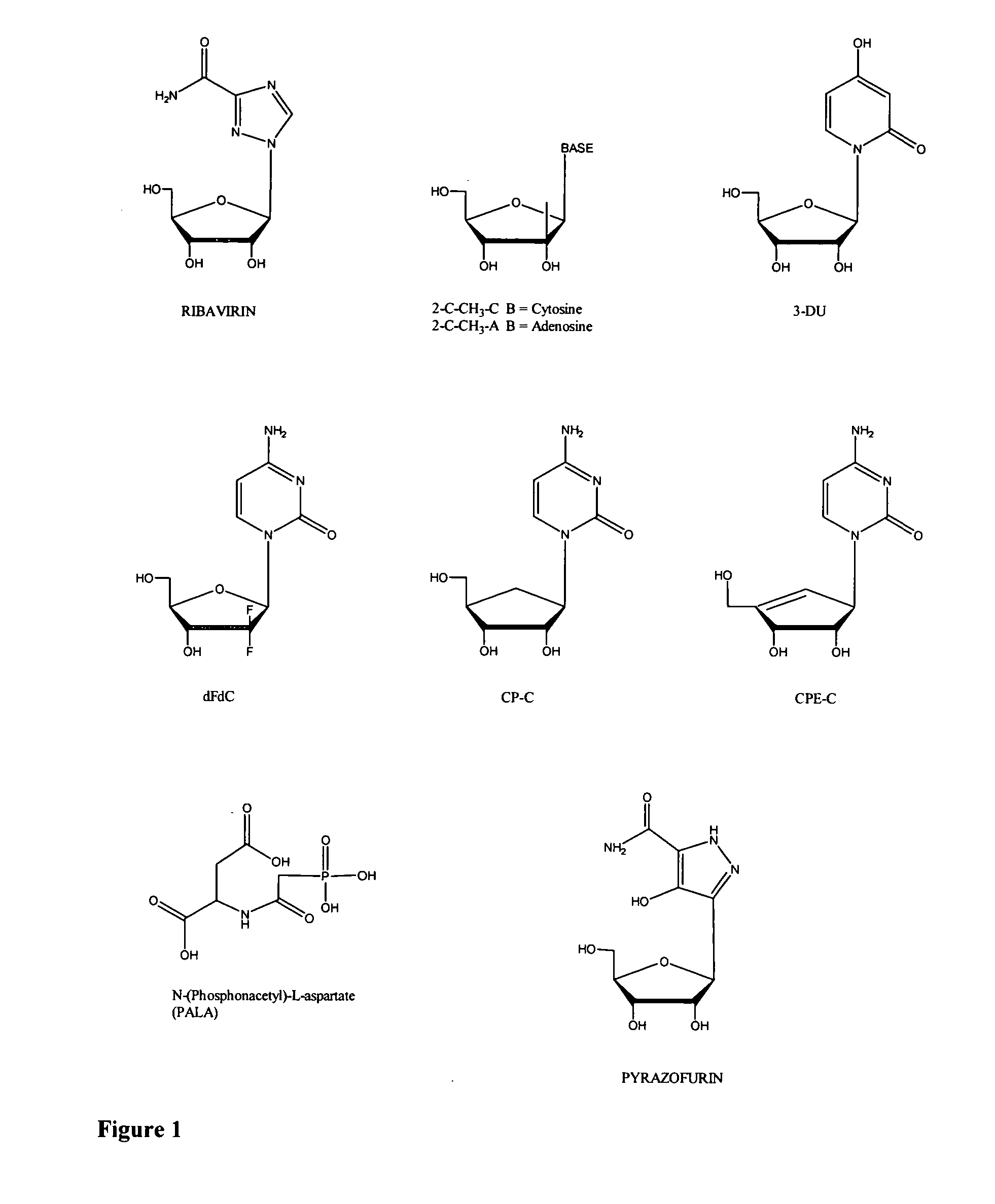
Infections with viruses of the family FLAVIVIRIDAE.
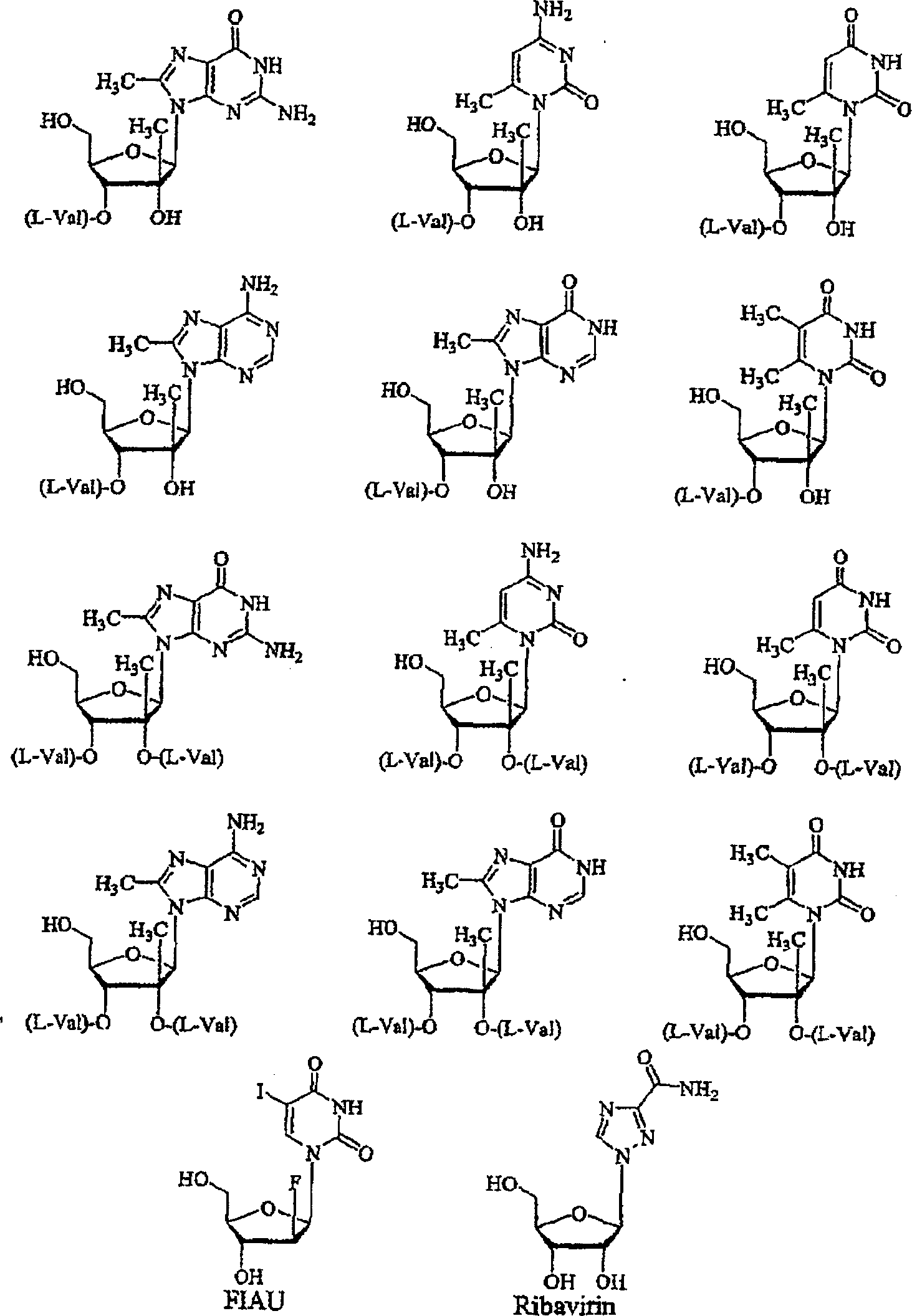
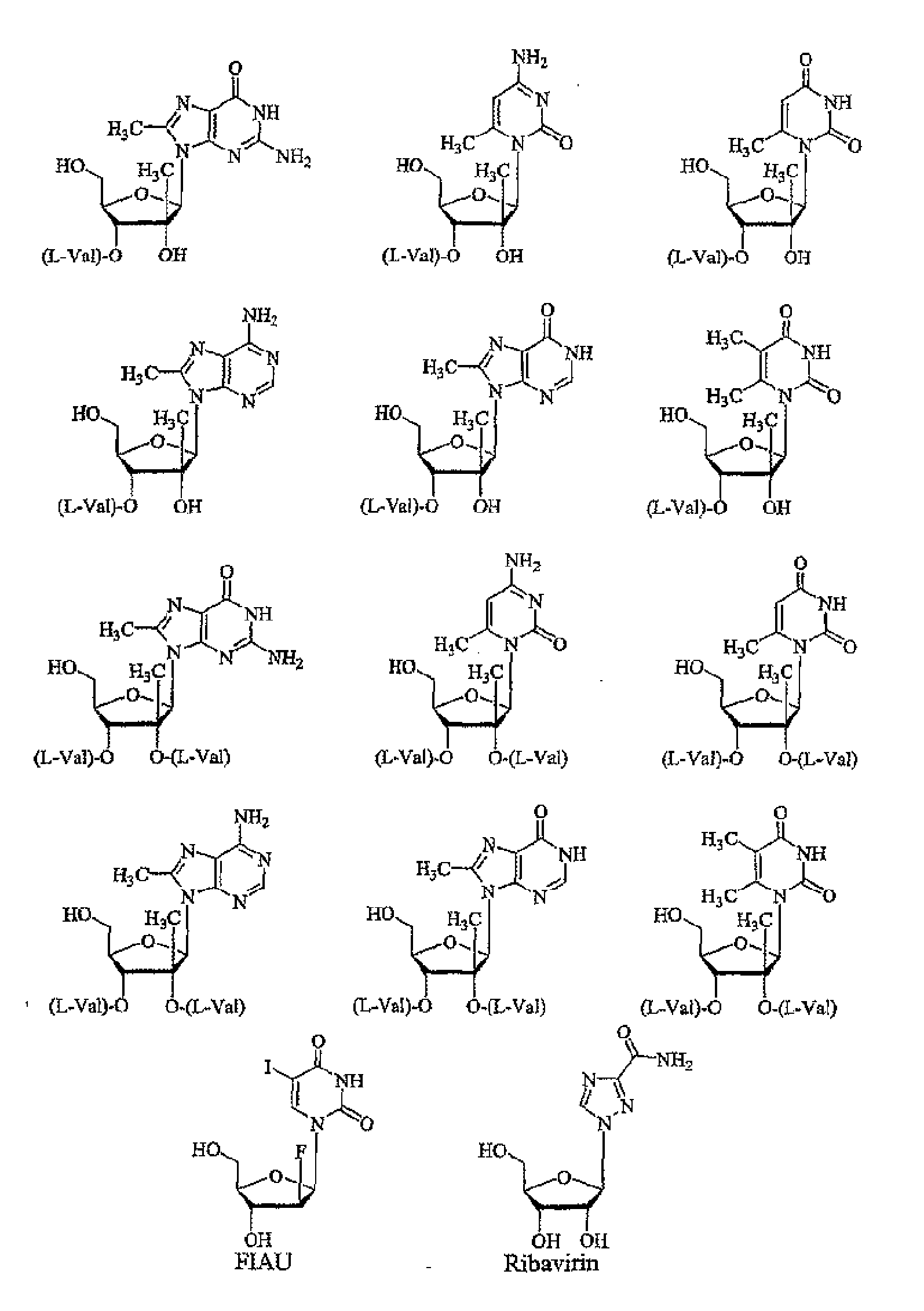
Modified fluorinated nucleoside analogues
ActiveUS20050009737A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
2' and 3'-nucleoside prodrugs for treating Flaviviridae infections
InactiveUS20070015905A1Inhibit HCV polymerase activityInhibit polymerase activityBiocideSugar derivativesDiseaseReverse transcriptase
2′ and 3′-Prodrugs of 1′, 2′, 3′ or 4′-branched β-D or β-L nucleosides, or their pharmaceutically acceptable salts and derivatives are described, which are useful in the prevention and treatment of Flaviviridae infections and other related conditions. These modified nucleosides provide superior results against flaviviruses and pestiviruses, including hepatitis C virus and viruses generally that replicate through an RNA dependent RNA reverse transcriptase. Compounds, compositions, methods and uses are provided for the treatment of Flaviviridae infection, including HCV infection, that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:INDENIX PHARM LLC +3
2' and 3'-nucleoside prodrugs for treating Flaviviridae infections
2′ and 3′-Prodrugs of 1′, 2′, 3′ or 4′-branched β-D or β-L nucleosides, or their pharmaceutically acceptable salts and derivatives are described, which are useful in the prevention and treatment of Flaviviridae infections and other related conditions. These modified nucleosides provide superior results against flaviviruses and pestiviruses, including hepatitis C virus and viruses generally that replicate through an RNA dependent RNA reverse transcriptase. Compounds, compositions, methods and uses are provided for the treatment of Flaviviridae infection, including HCV infection, that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:THE CENT NAT DEL LA RECH SCIQUE +3
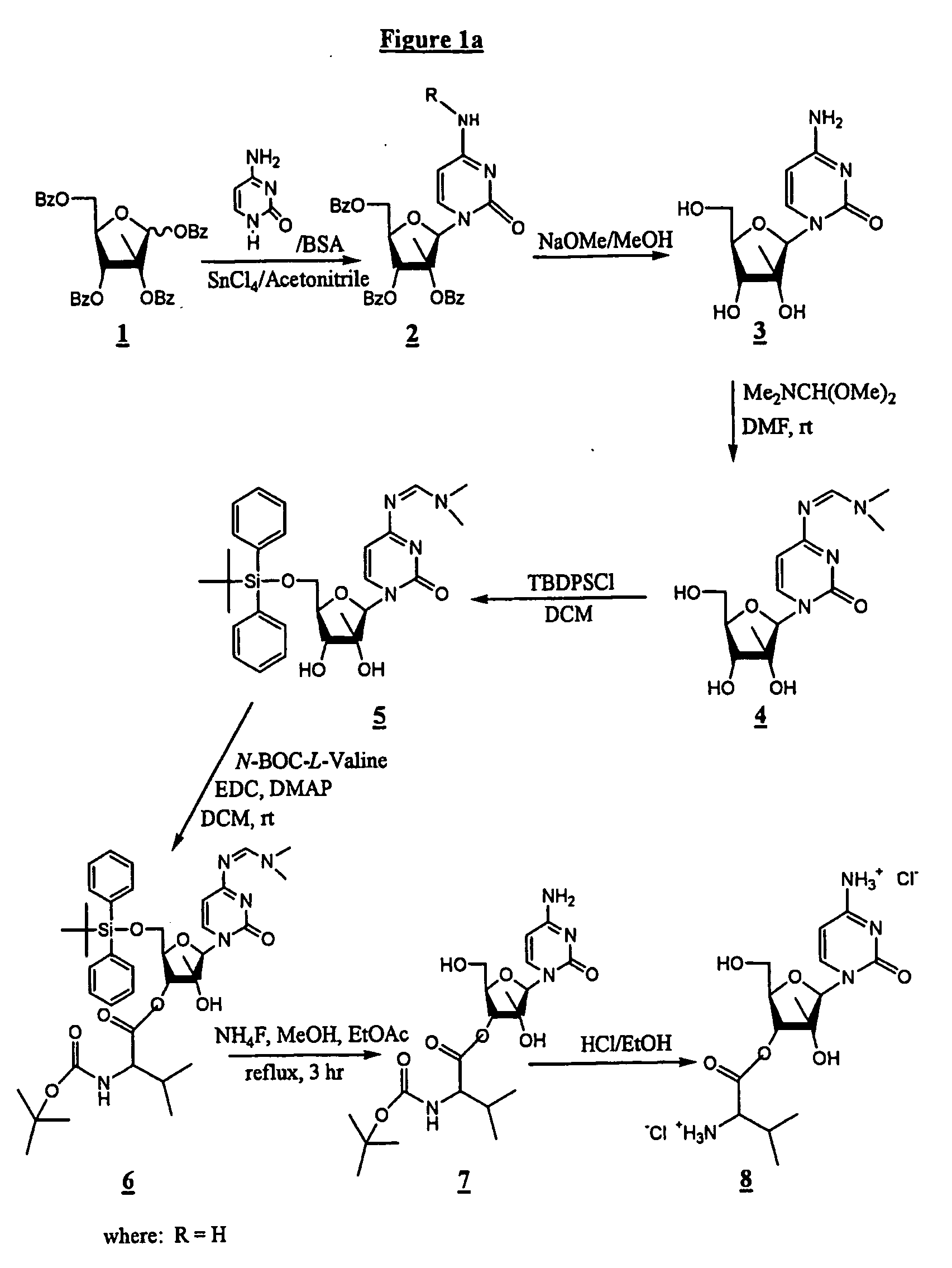
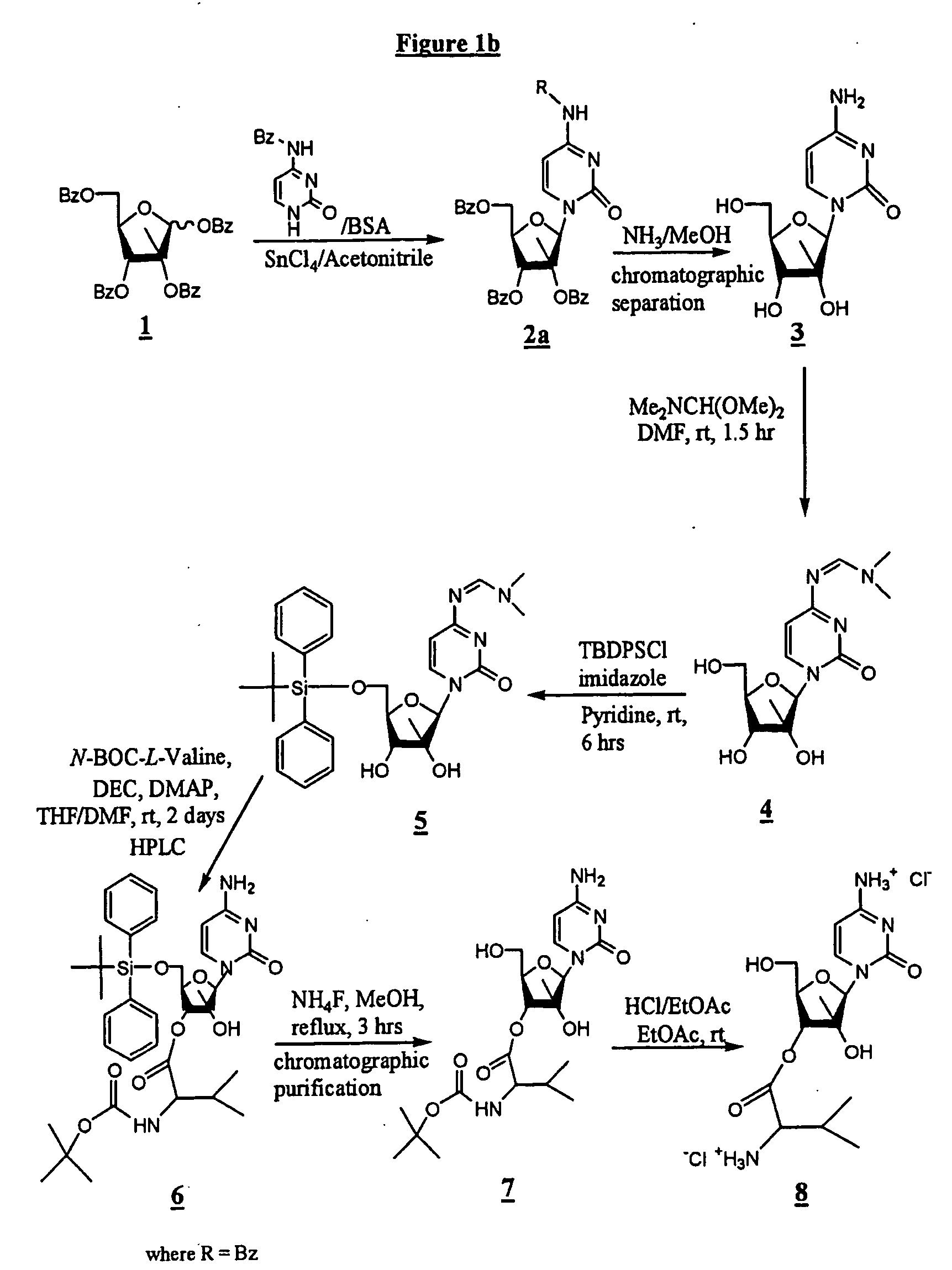
2'-C-methyl-3'-O-L-valine ester ribofuranosyl cytidine for treatment of flaviviridae infections
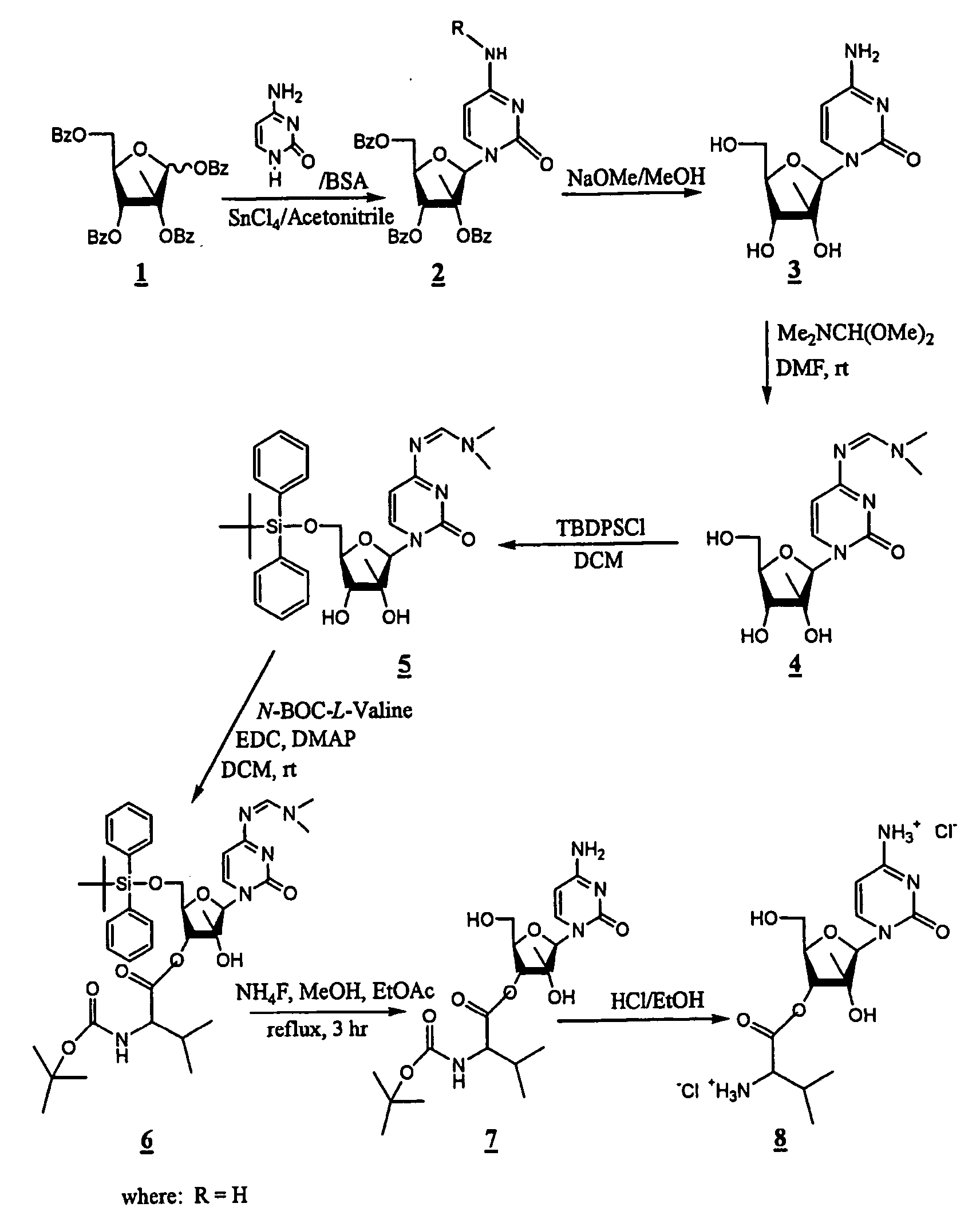
The 3′-L-valine ester of β-D-2′-C-methyl-ribofuranosyl cytidine provides superior results against flaviviruses and pestiviruses, including hepatitis C virus. Based on this discovery, compounds, compositions, methods and uses are provided for the treatment of flaviviridae, including HCV, that include the administration of an effective amount of val-mCyd or its salt, ester, prodrug or derivative, optionally in a pharmaceutically acceptable carrier. In an alternative embodiment, val-mCyd is used to treat any virus that replicates through an RNA-dependent RNA polymerase.
Owner:INDENIX PHARM LLC +3
Modified fluorinated nucleoside analogues
ActiveUS20080070861A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
2′-branched nucleosides and Flaviviridae mutation
ActiveUS7824851B2High sensitivityReduce Flaviviridae infectionBiocideSsRNA viruses positive-senseAmino acidMutant strain
The present invention discloses a method for the treatment of Flaviviridae infection that includes the administration of a 2′-branched nucleoside, or a pharmaceutically acceptable prodrug and / or salt thereof, to a human in need of therapy in combination or alternation with a drug that directly or indirectly induces a mutation in the viral genome at a location other than a mutation of a nucleotide that results in a change from seine to a different amino acid in the highly conserved consensus sequence, XRXSGXXXT (Sequence ID No. 63), of domain B of the RNA polymerase region, or is associated with such a mutation. The invention also includes a method to detect a mutant strain of Flaviviridae and a method for its treatment.
Owner:INDENIX PHARM LLC
Antiviral agents for treatment of Flaviviridae infections
InactiveUS20040266723A1Alleviating and preventing and delaying onsetEffective conditioningBiocideSugar derivativesPestivirusMedicine
The disclosed invention is a composition for and a method of treating Flaviviridae (Hepacivirus, Flavivirus, Pestivirus) infections, including BVDV and HCV, in a host, including animals, and especially humans, using a small molecule or its pharmaceutically acceptable salt or prodrug.
Owner:PHARMASSET
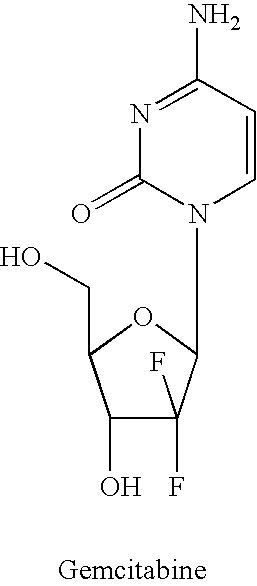
Dosing regimen for gemcitabine HCV therapy
InactiveUS20030225029A1Reduce viral loadRapid and large in viral loadBiocideSugar derivativesDosing regimenHepatitis c viral
A dosage regiment for the treatment of a Flaviviridae infection, including a hepatitis C viral infection, that includes administering gemcitabine (or its salt, prodrug or derivative, as described herein) in a dosage range of approximately 50 mg / m<2 >to about 1300 mg / m<2 >per day for between one and seven days (e.g. 1, 2, 3, 4, 5, 6, or 7 days) followed by cessation of therapy. Viral load is optionally monitored over time, and after cessation, viral rebound is monitored. Therapy is not resumed unless a significant viral load is again observed, and then therapy for 1-7 days and more preferred, 1, 2 or 3 days, is repeated. This therapy can be continued indefinitely to monitor and maintain the health of the patient.
Owner:PHARMASSET
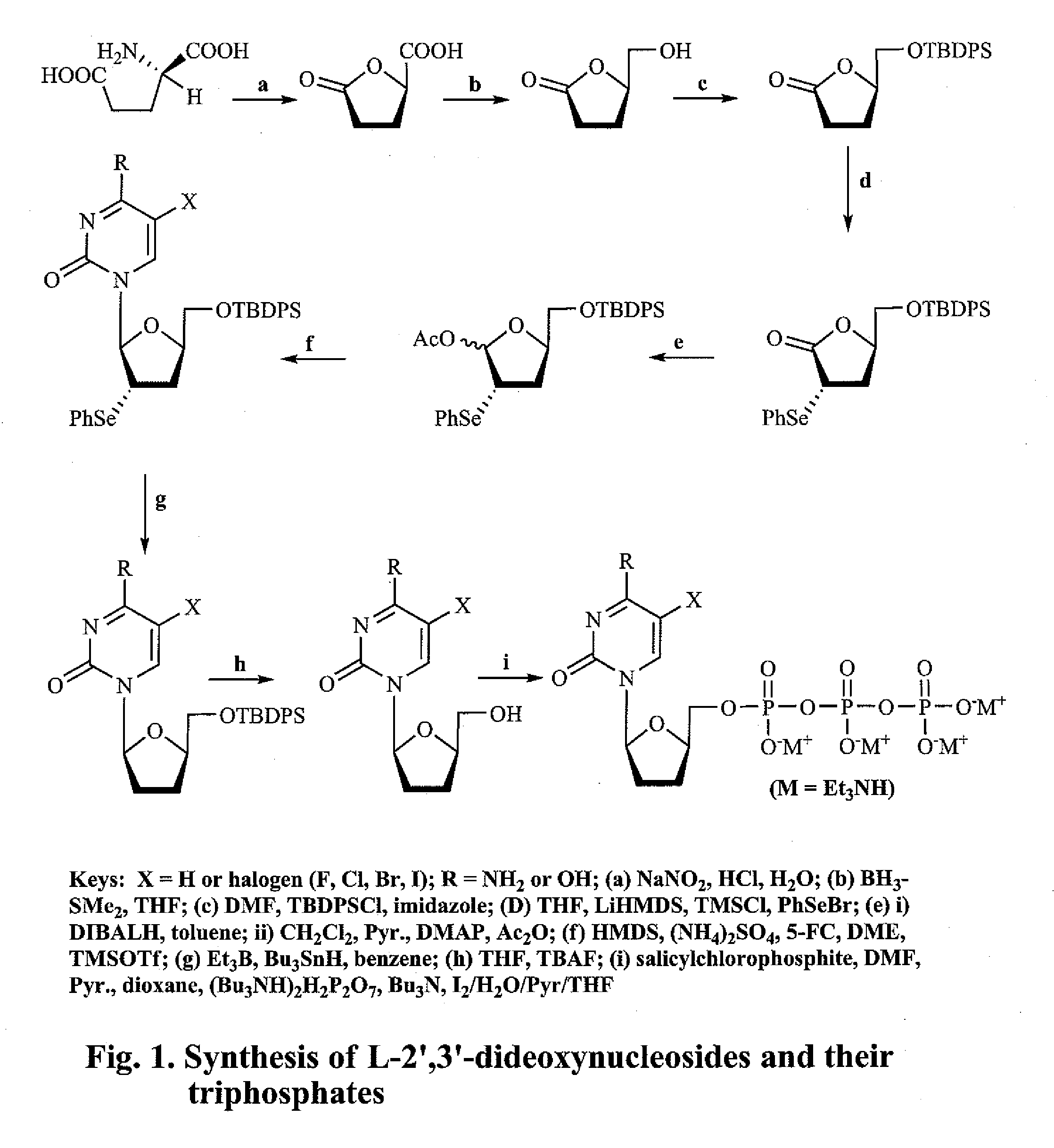
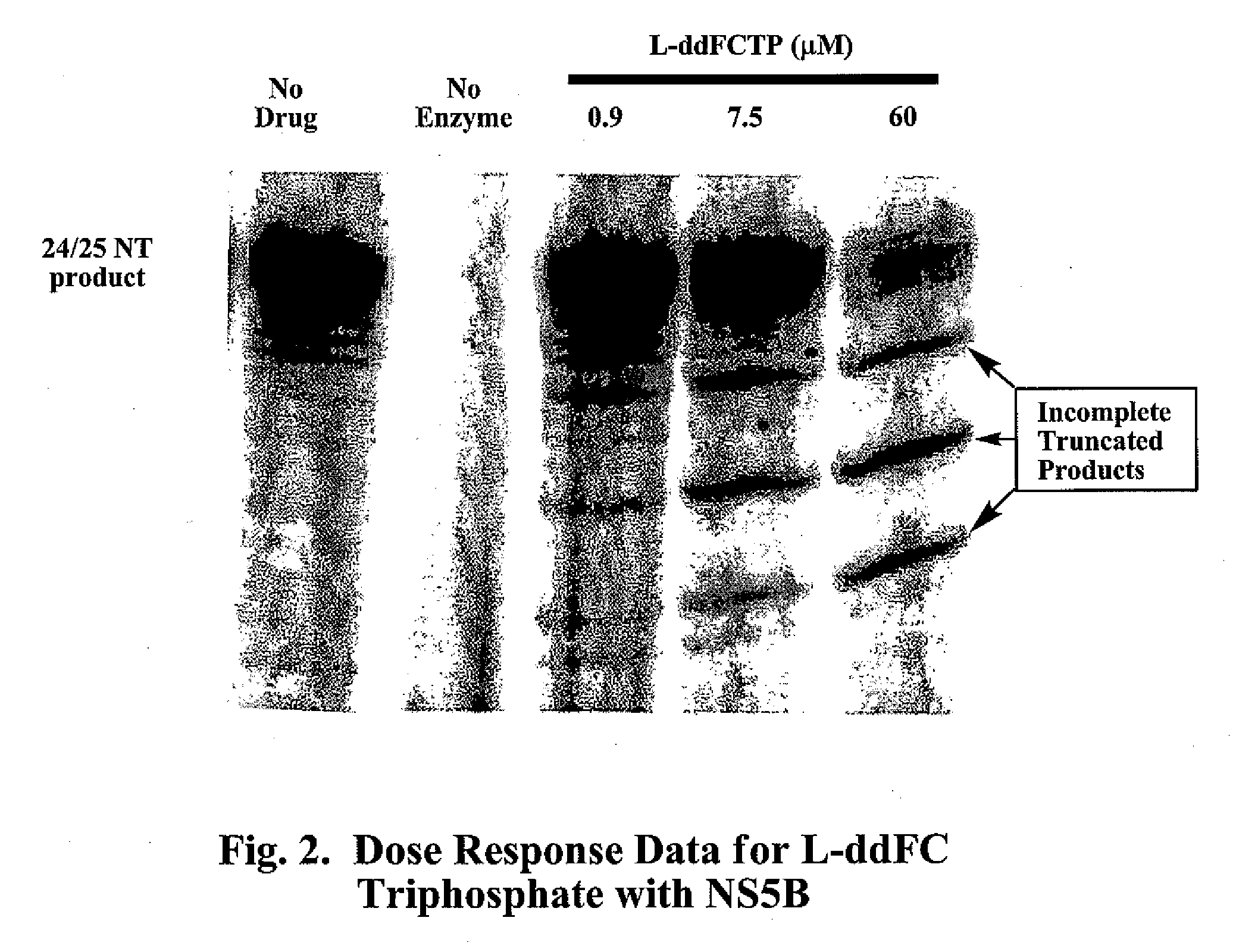
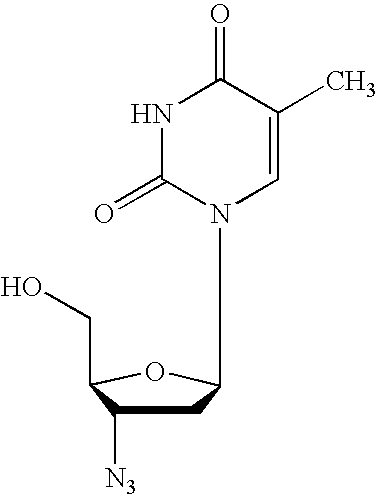
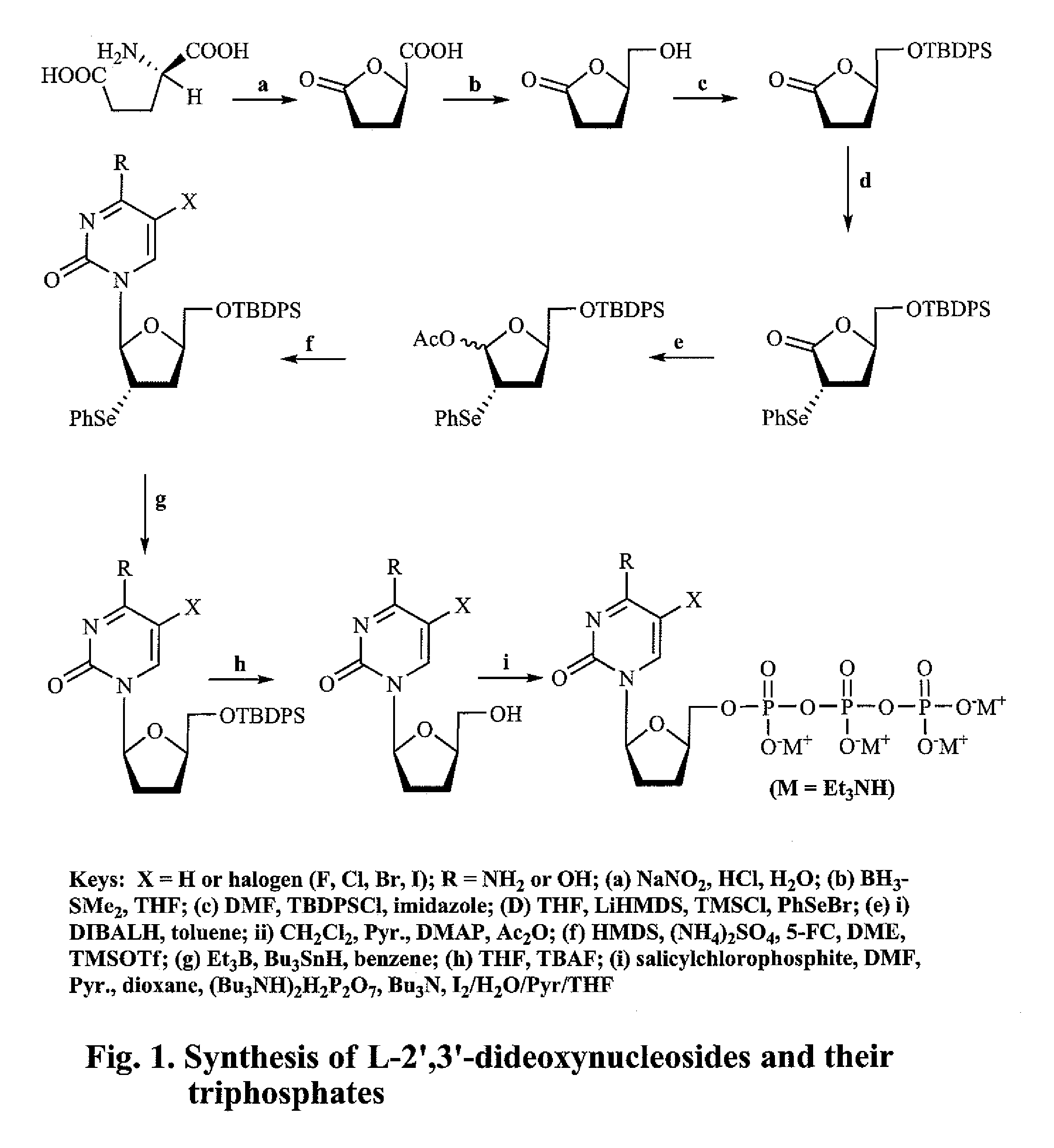
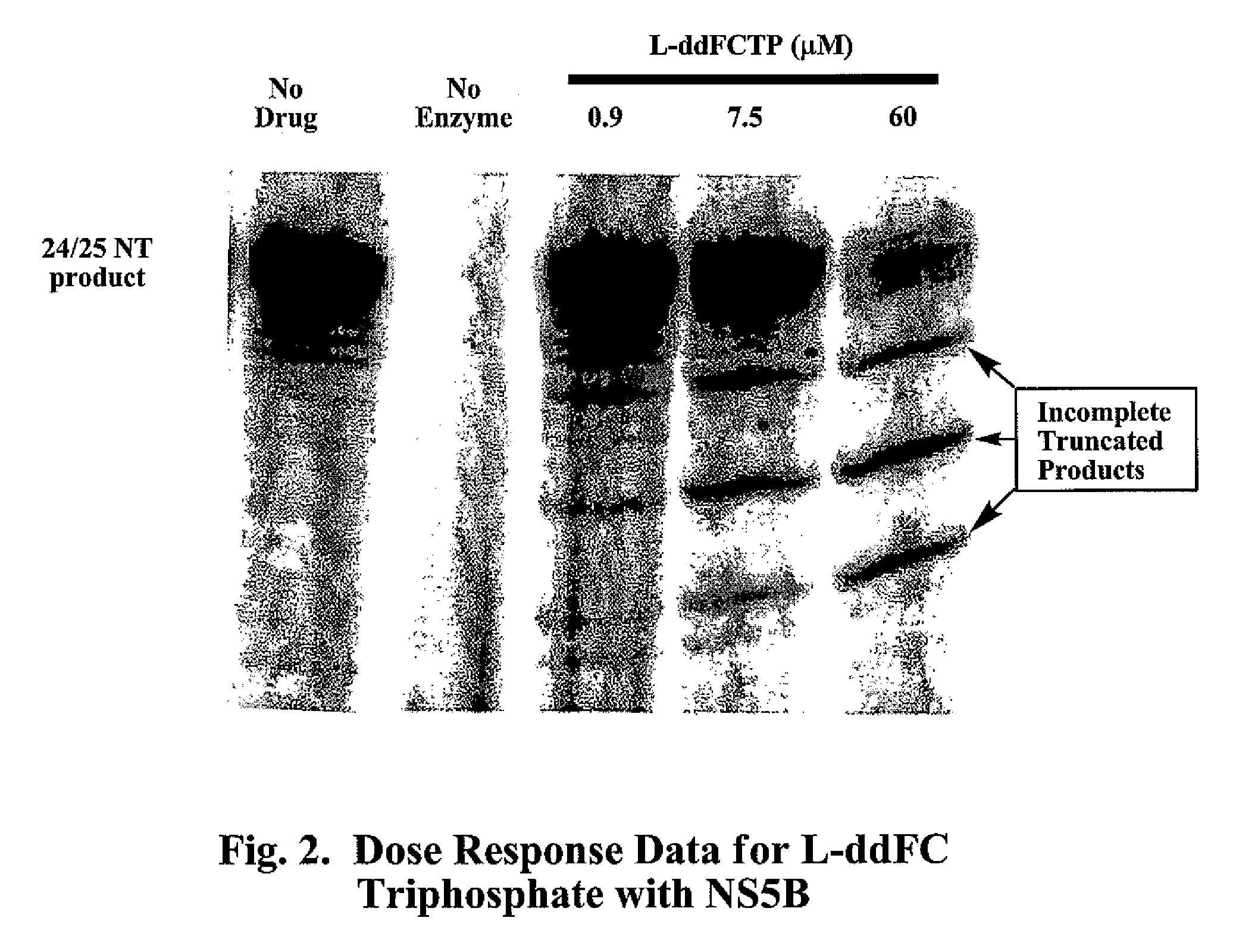
2′,3′-dideoxynucleoside analogues for the treatment or prevention of Flaviviridae infections
A method for the treatment or prevention of Flaviviridae infections, in particular, hepatitis C virus infection, in a host, and in particular, a human, is provided that includes administering an effective amount of a β-L- or β-D-2′,3′-dideoxynucleoside or a pharmaceutically acceptable salt or prodrug thereof, optionally in a pharmaceutically acceptable diluent or excipient.
Owner:GILEAD SCI INC
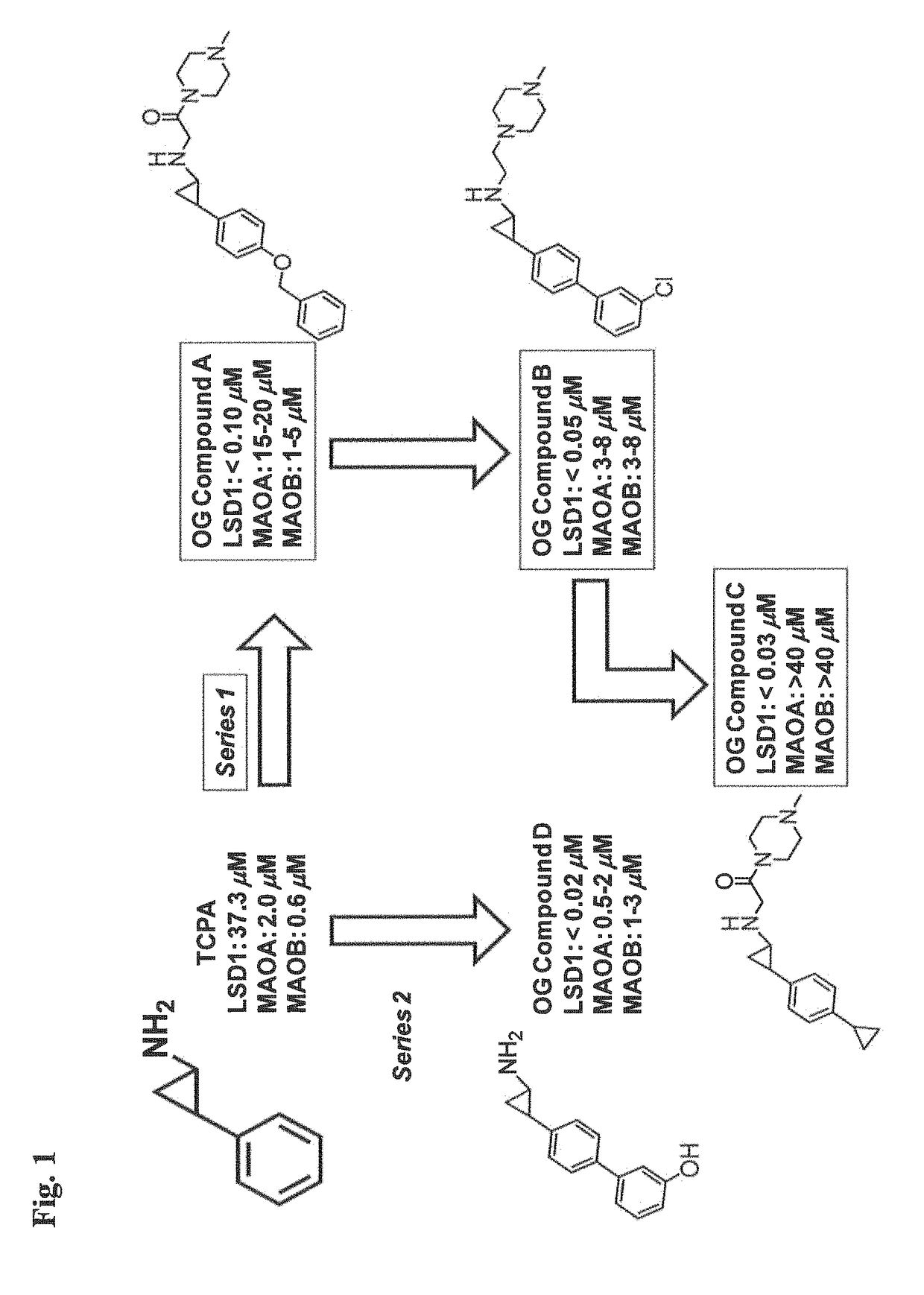
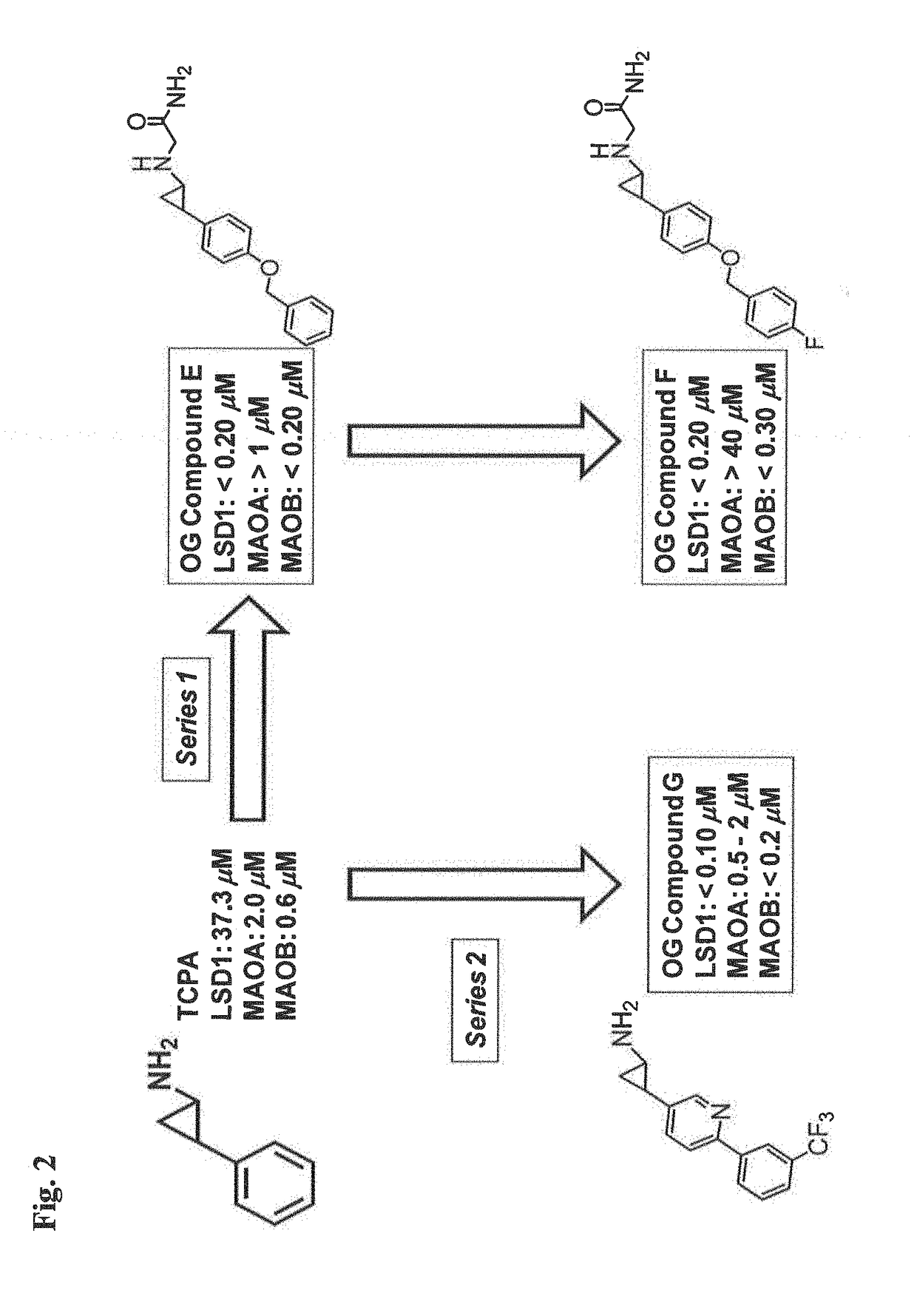
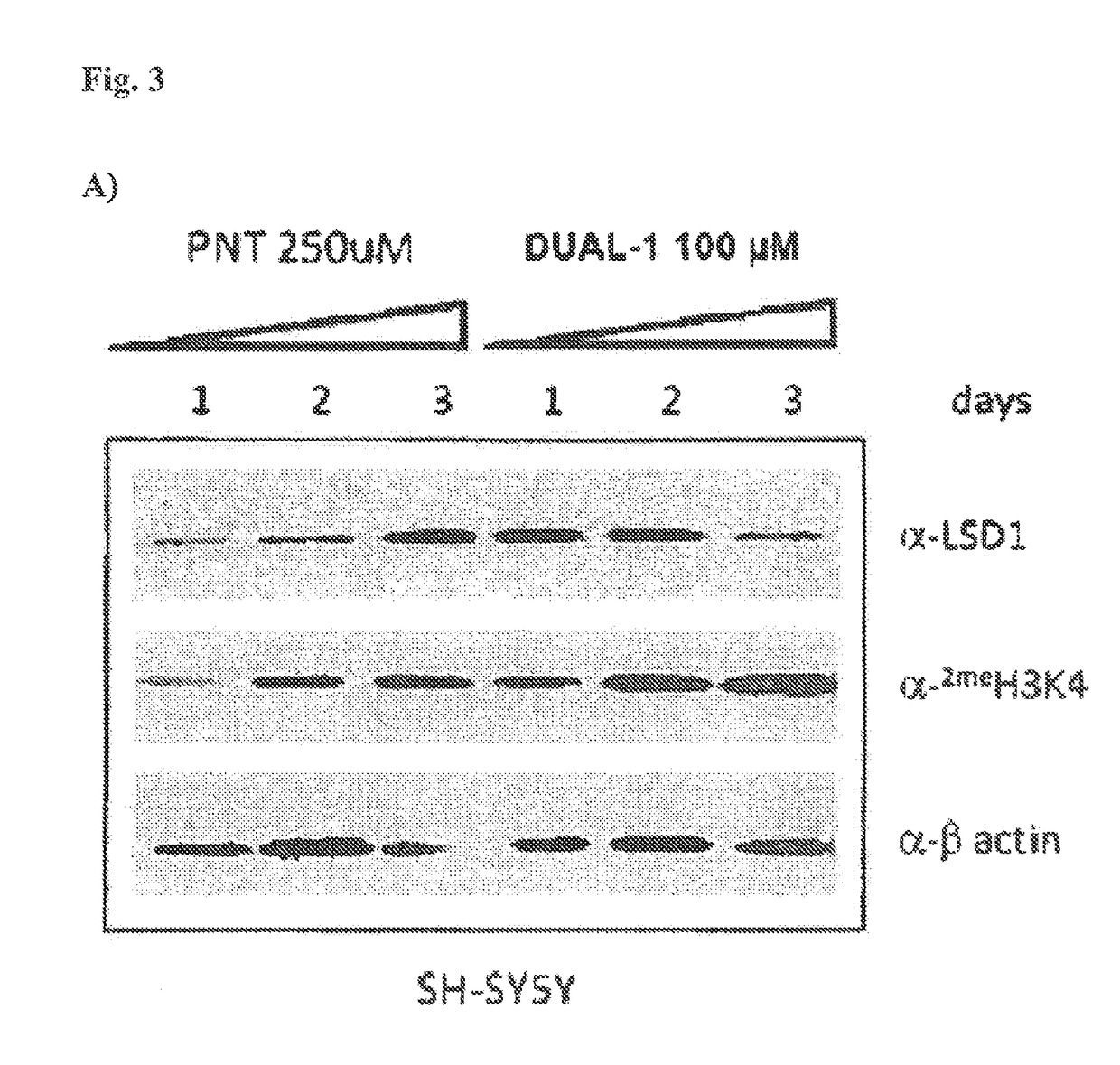
Lysine demethylase inhibitors for diseases and disorders associated with flaviviridae
ActiveUS20140256742A1Avoids side-effectsReduce duplicationBiocideOrganic active ingredientsLysineLuetic disease
Owner:ORYZON GENOMICS SA
Modified 2' and 3'-nucleoside prodrugs for treating Flaviviridae infections
InactiveUS20070027065A1Inhibit Flaviviridae polymerase activityInhibit polymerase activityBiocideAntiviralsNucleoside XProdrug
2′ and / or 3′ prodrugs of 1′, 2′, 3′ or 4′-branched nucleosides, and their pharmaceutically acceptable salts and derivatives are described. These prodrugs are useful in the prevention and treatment of Flaviviridae infections, including HCV infection, and other related conditions. Compounds and compositions of the prodrugs of the present invention are described. Methods and uses are also provided that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:THE CENT NAT DEL LA RECH SCIQUE +3
Modified 2' and 3'-nucleoside prodrugs for treating flaviviridae infections
InactiveUS20070032449A1Prevent and retard progressionDetermine the spectrum of activityBiocideSugar derivativesNucleoside XNucleoside
2′ and / or 3′ prodrugs of 1′, 2′, 3′ or 4′-branched nucleosides, and their pharmaceutically acceptable salts and derivatives are described. These prodrugs are useful in the prevention and treatment of Flaviviridae infections, including HCV infection, and other related conditions. Compounds and compositions of the prodrugs of the present invention are described. Methods and uses are also provided that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:THE CENT NAT DEL LA RECH SCIQUE +3
Modified 2' and 3'-nucleoside prodrugs for treating Flaviviridae infections
InactiveUS20070027104A1Inhibit Flaviviridae polymerase activityInhibit polymerase activityBiocideSugar derivativesNucleoside XProdrug
2′ and / or 3′ prodrugs of 1′, 2′, 3′ or 4′-branched nucleosides, and their pharmaceutically acceptable salts and derivatives are described. These prodrugs are useful in the prevention and treatment of Flaviviridae infections, including HCV infection, and other related conditions. Compounds and compositions of the prodrugs of the present invention are described. Methods and uses are also provided that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:THE CENT NAT DEL LA RECH SCIQUE +3
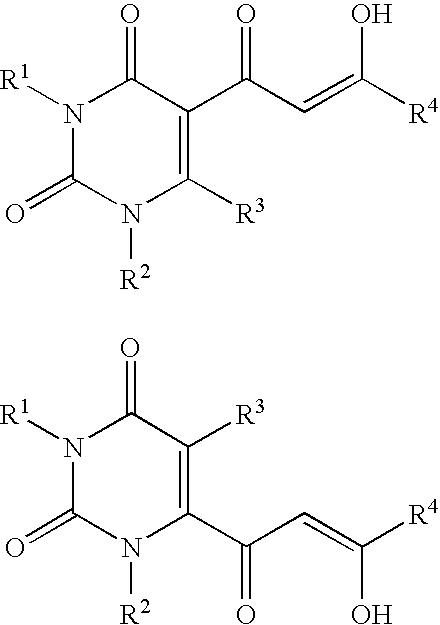
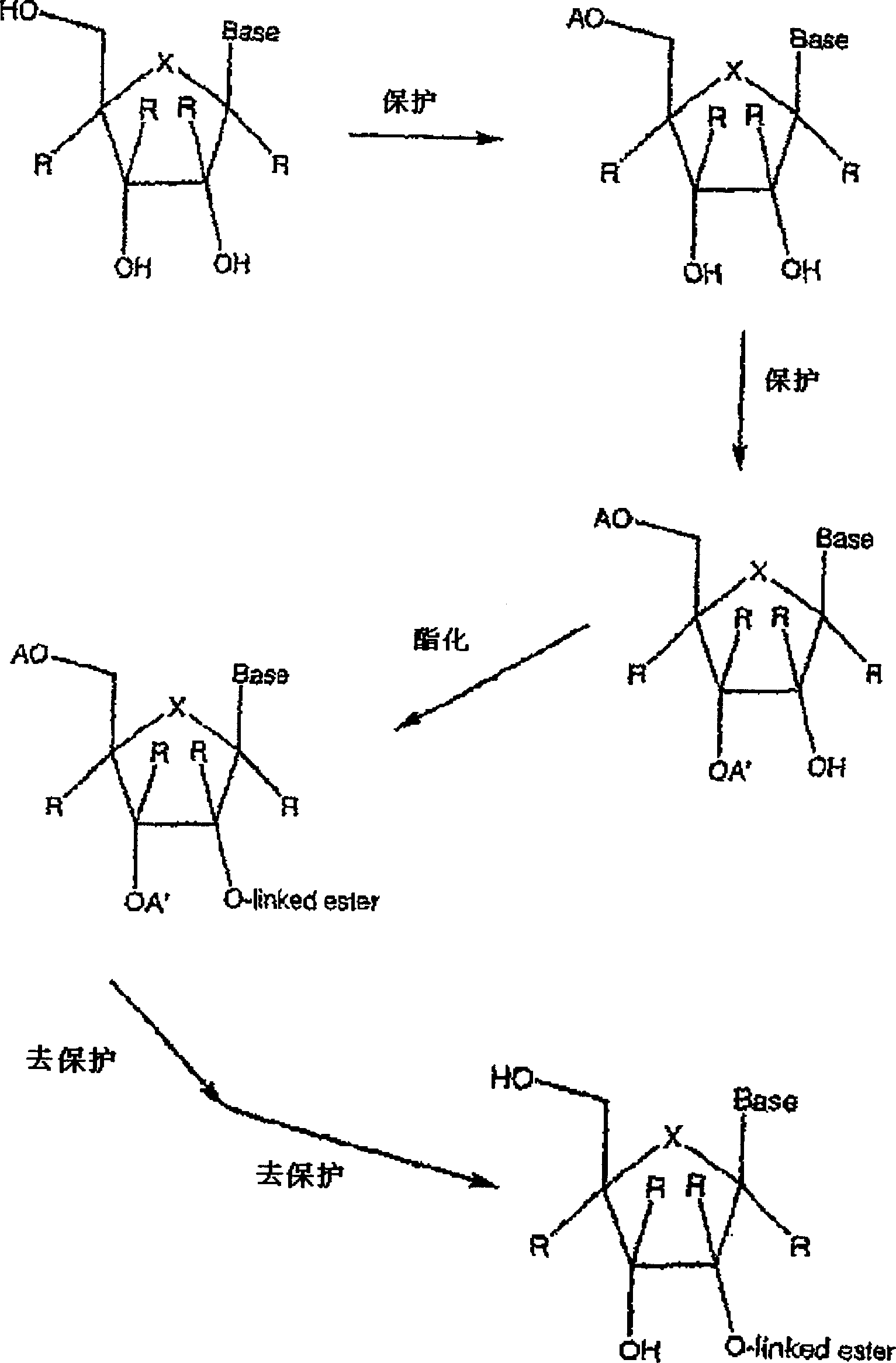
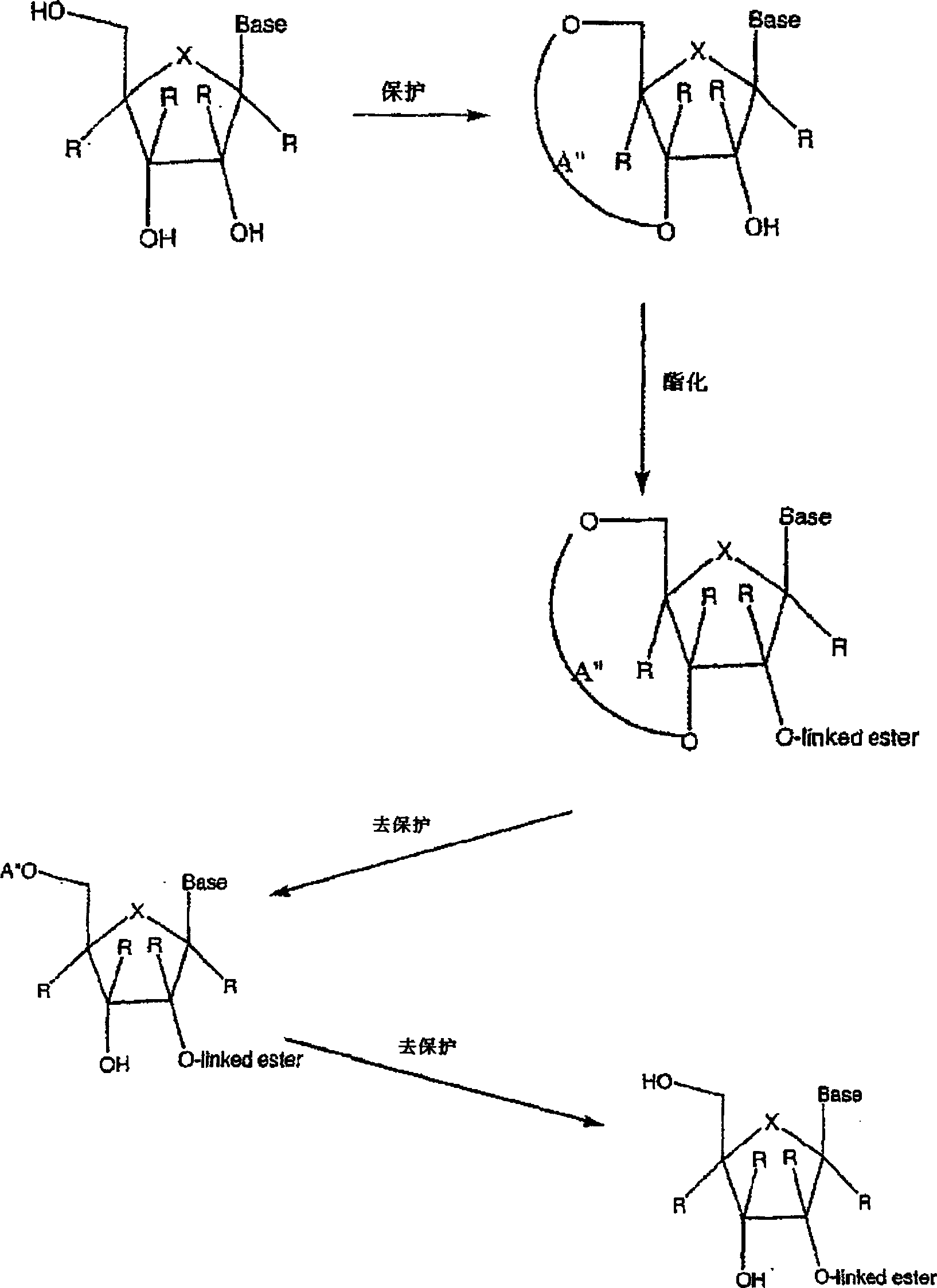
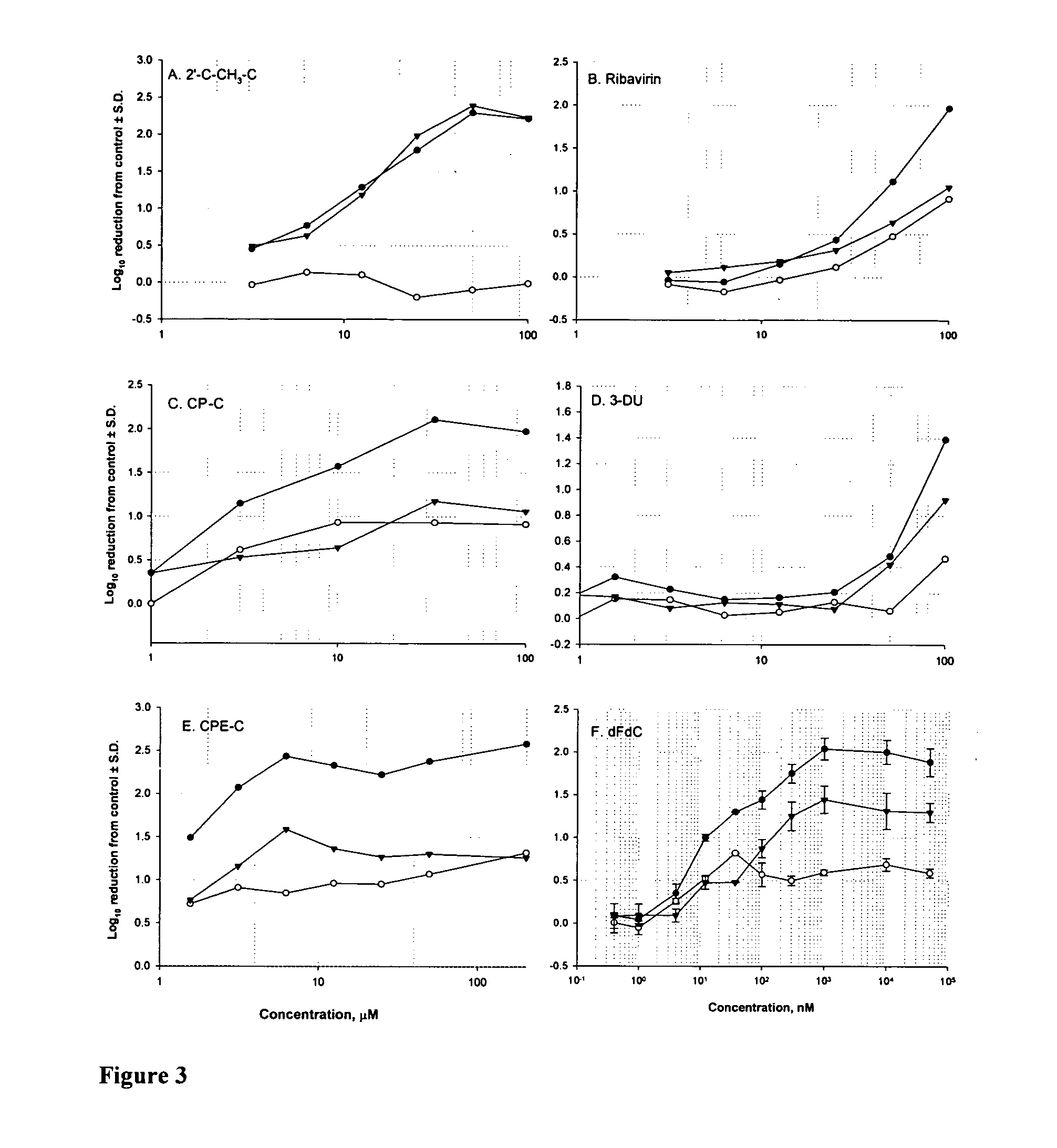
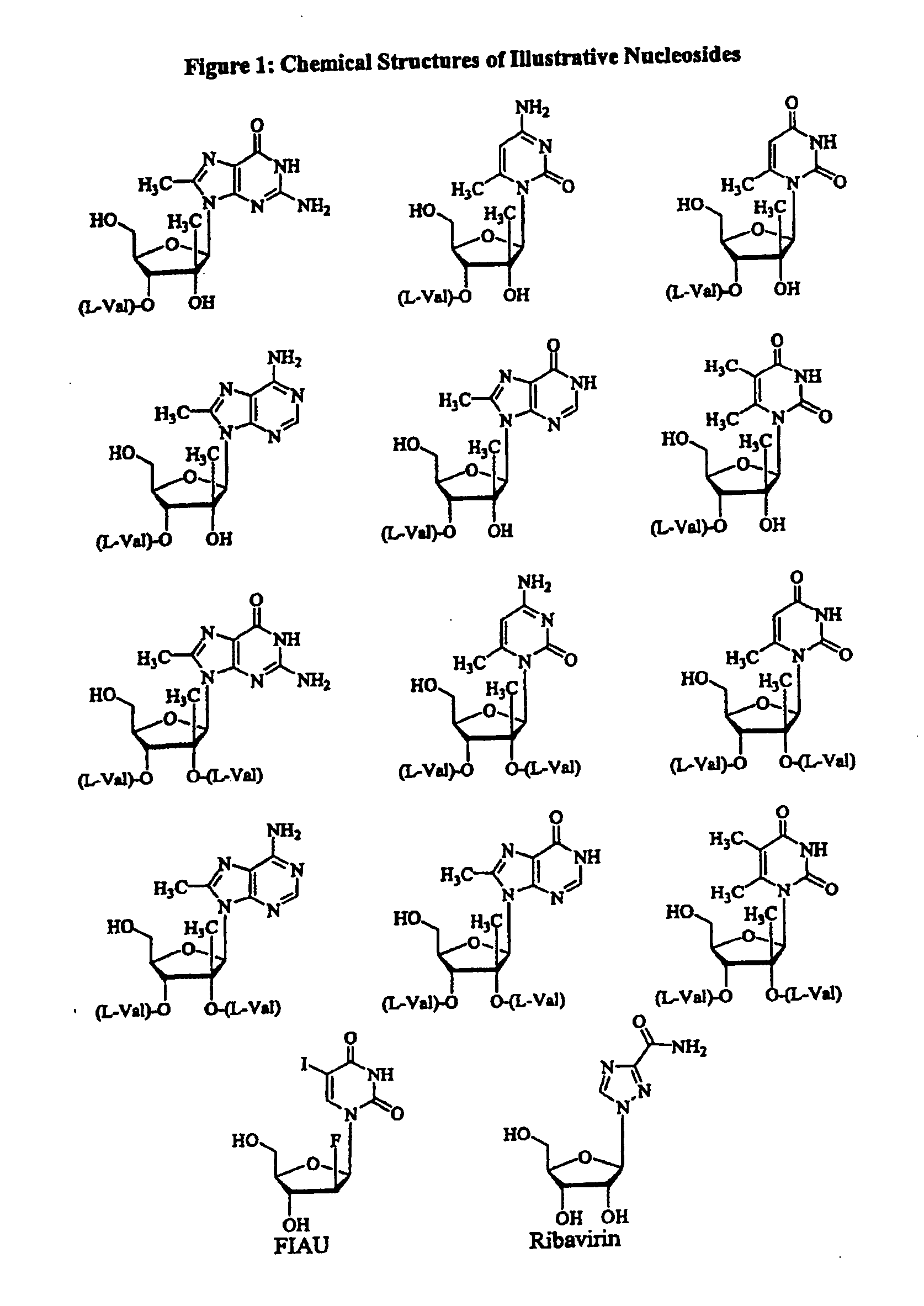
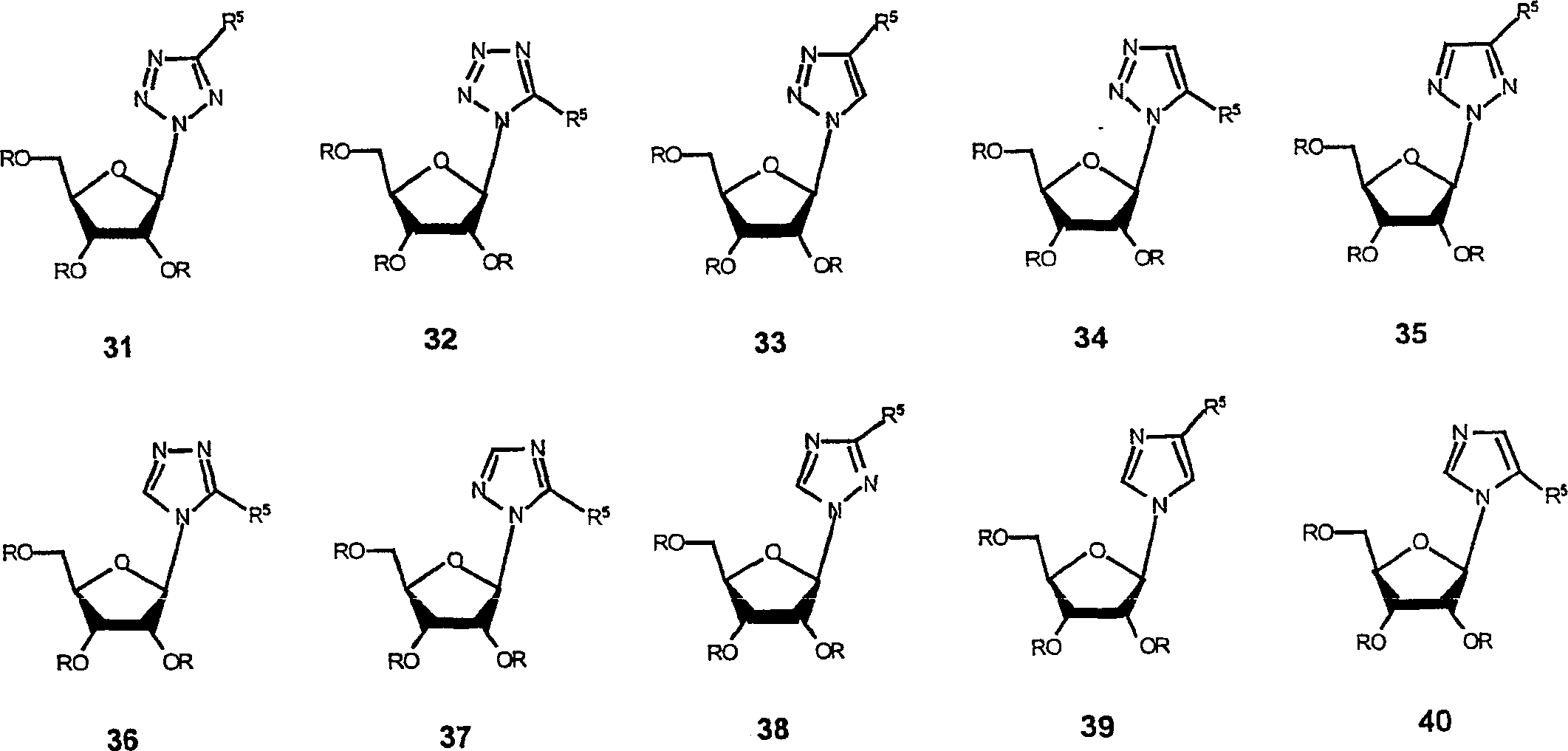
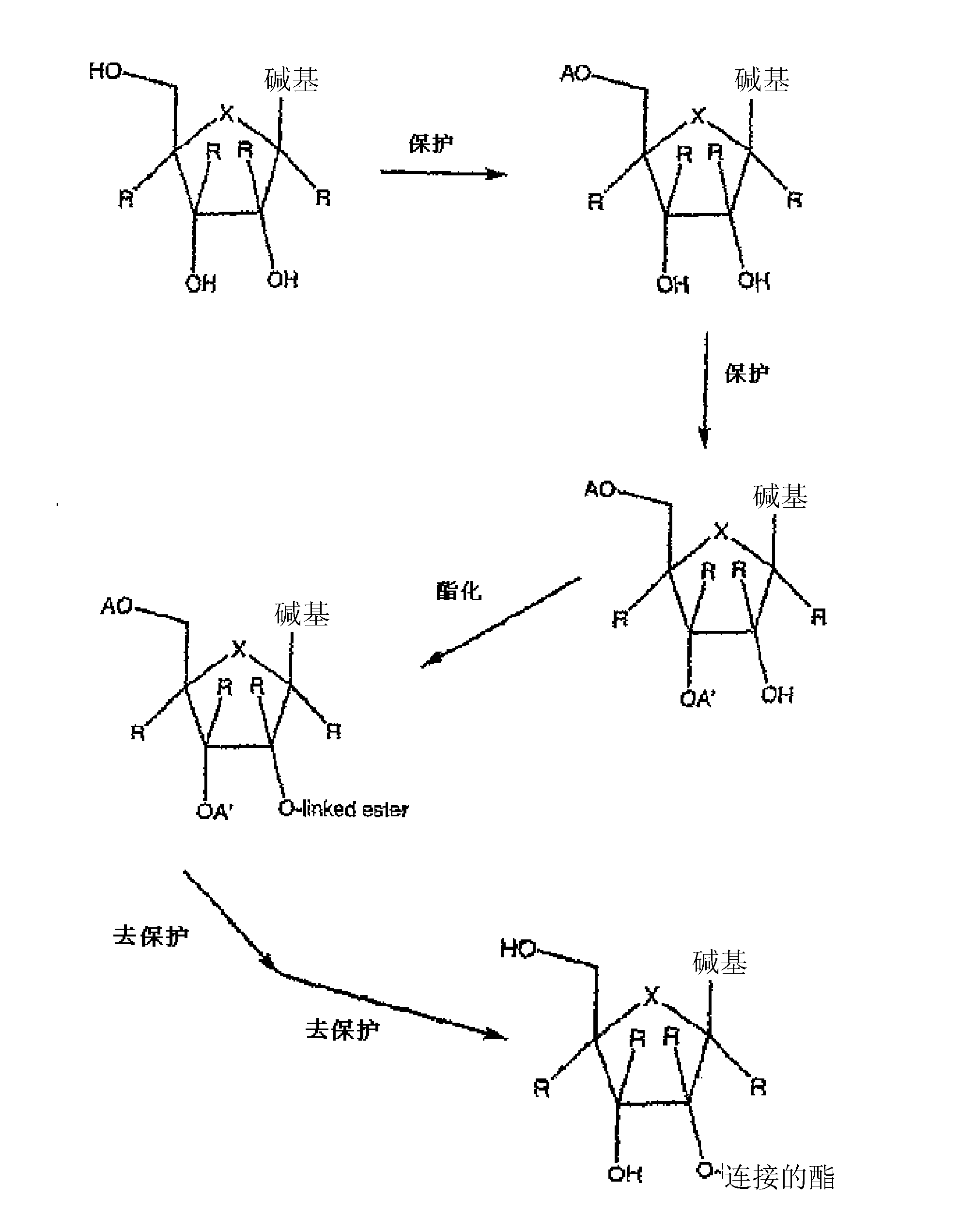
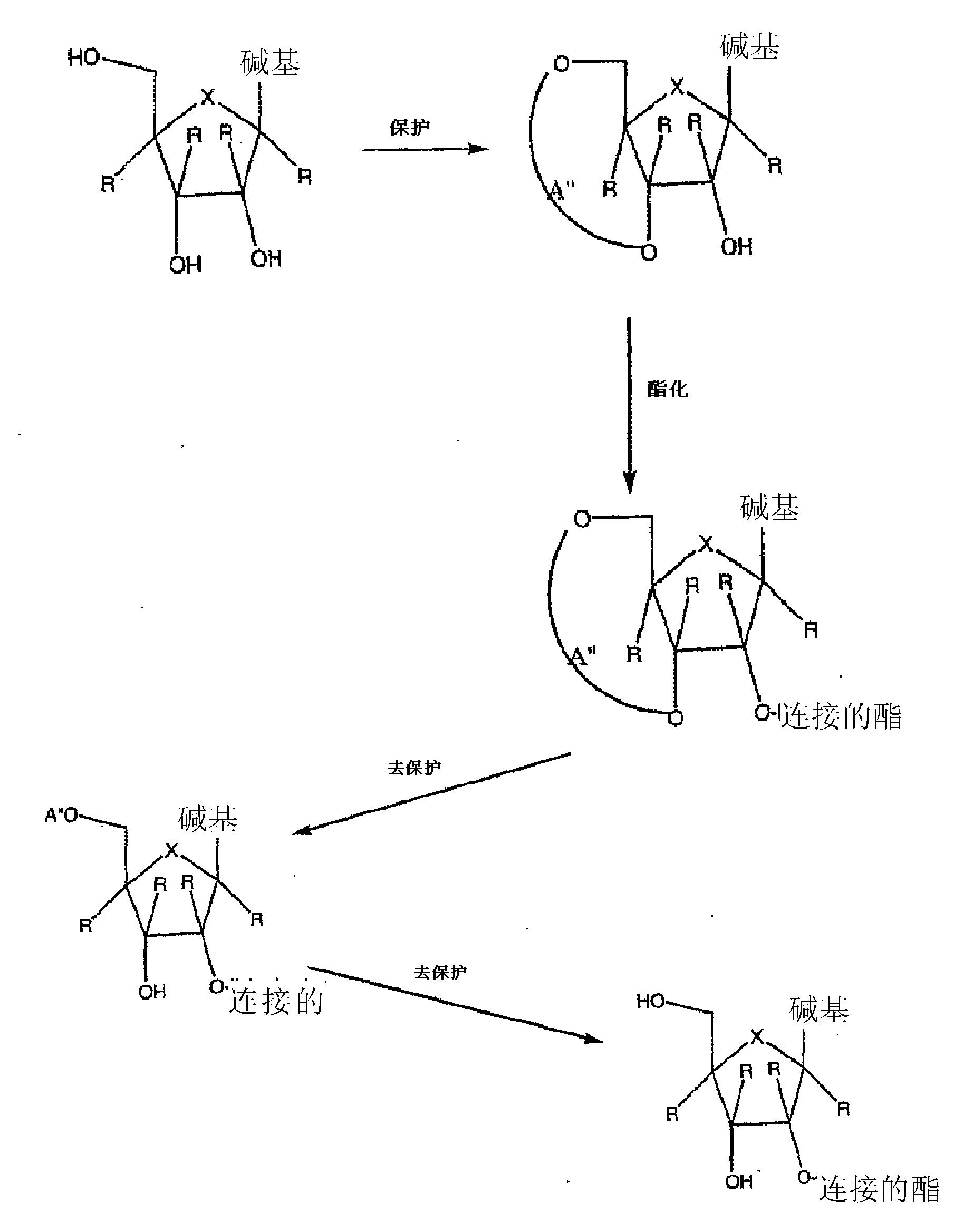
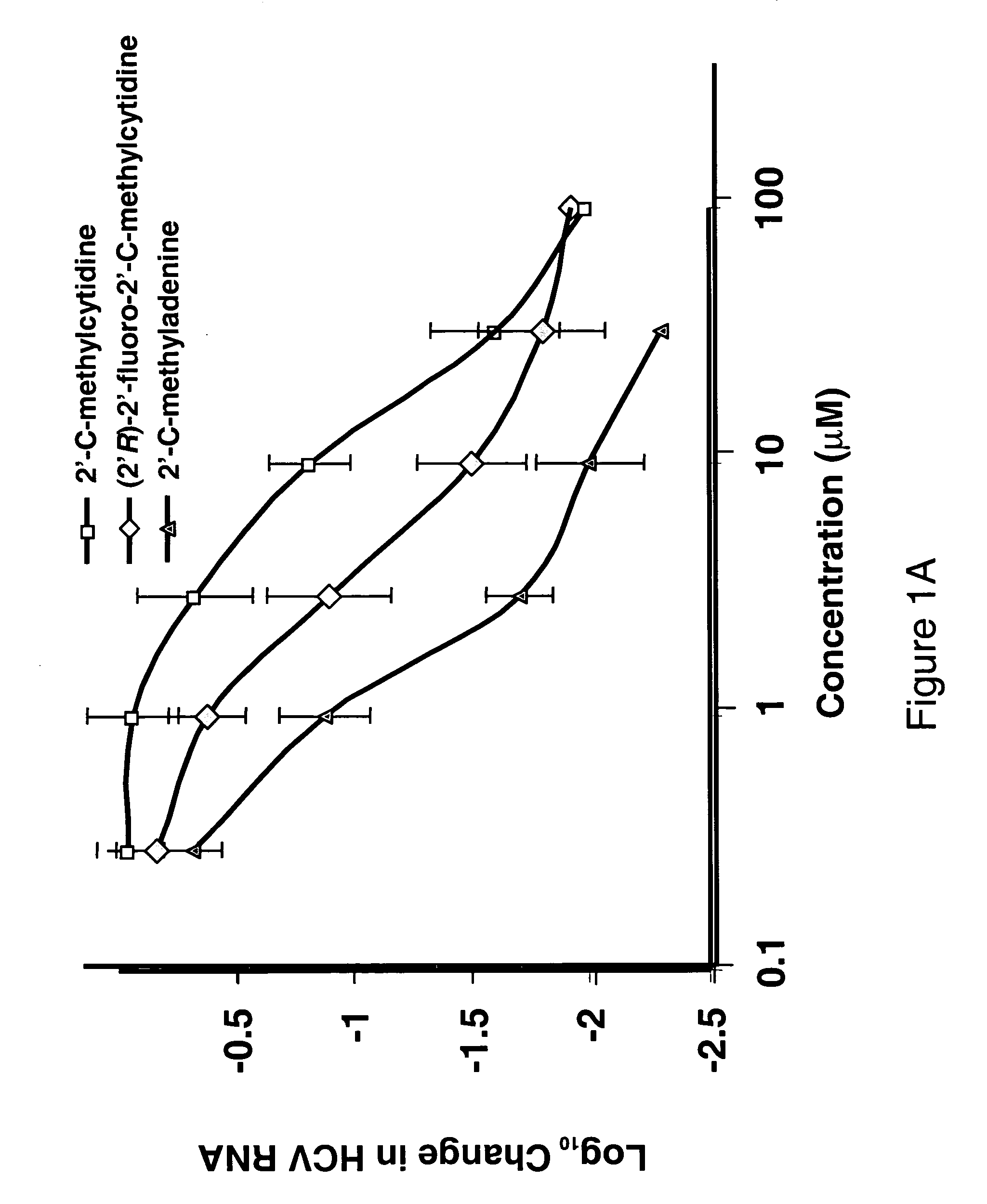
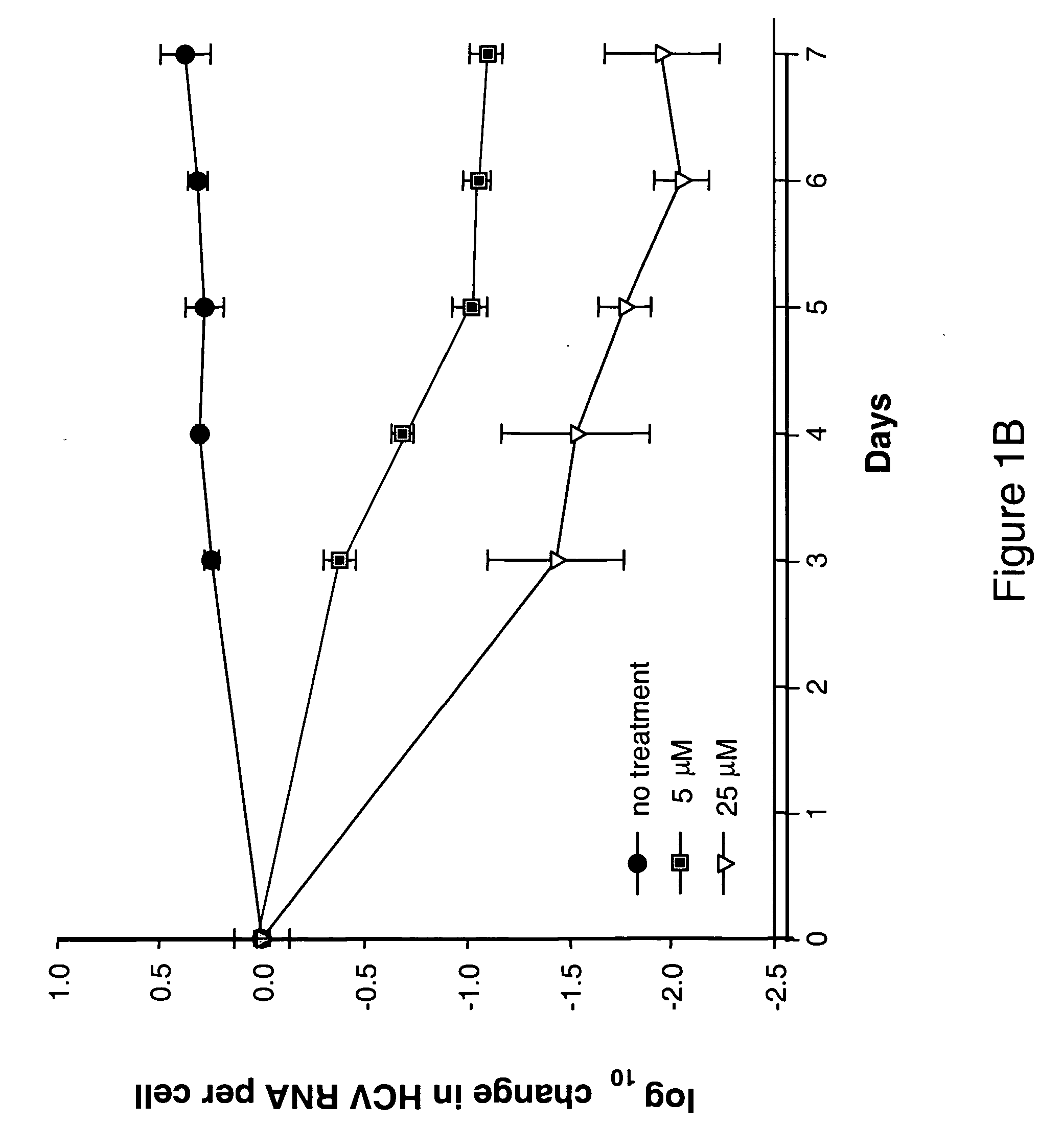
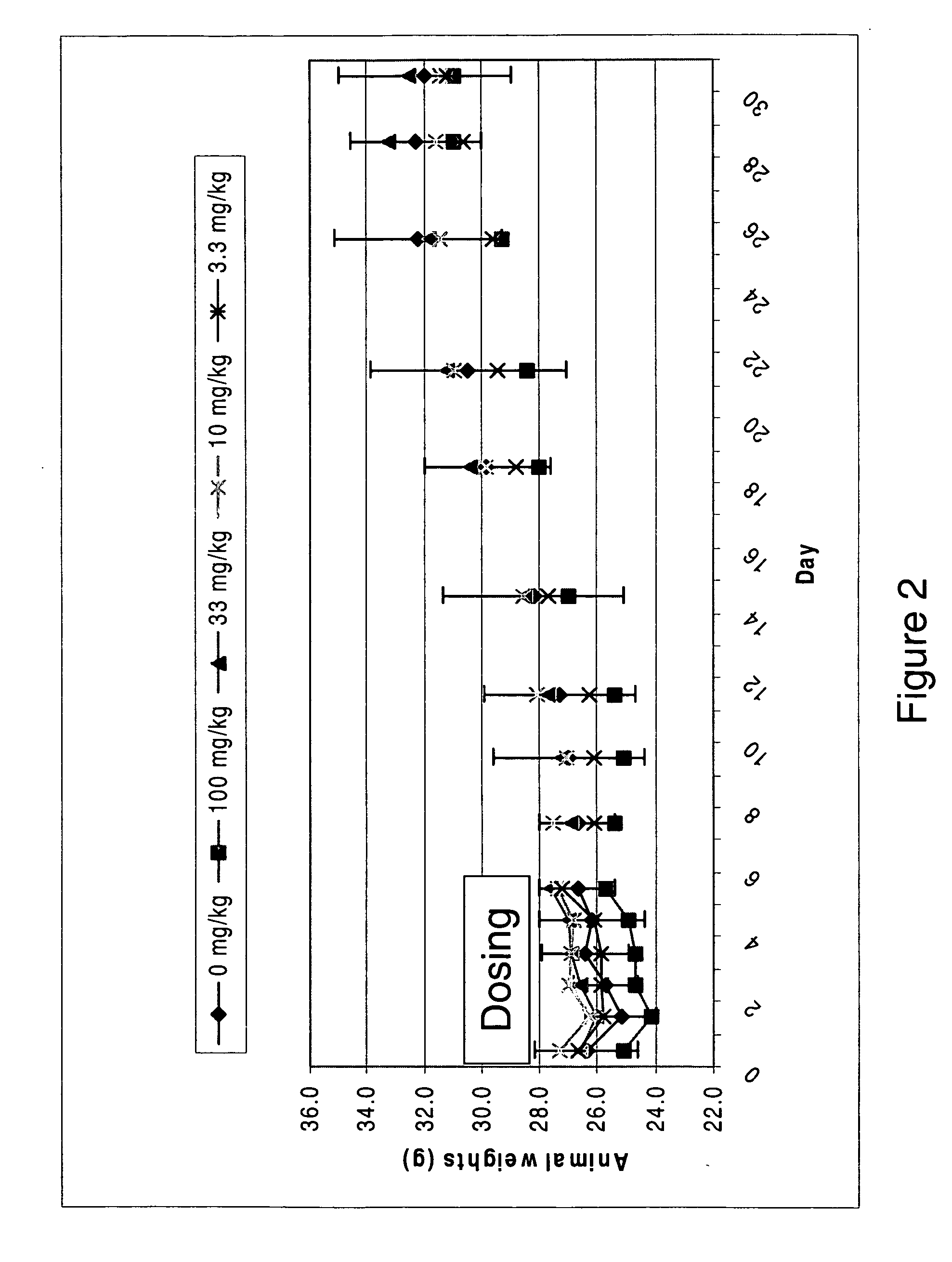
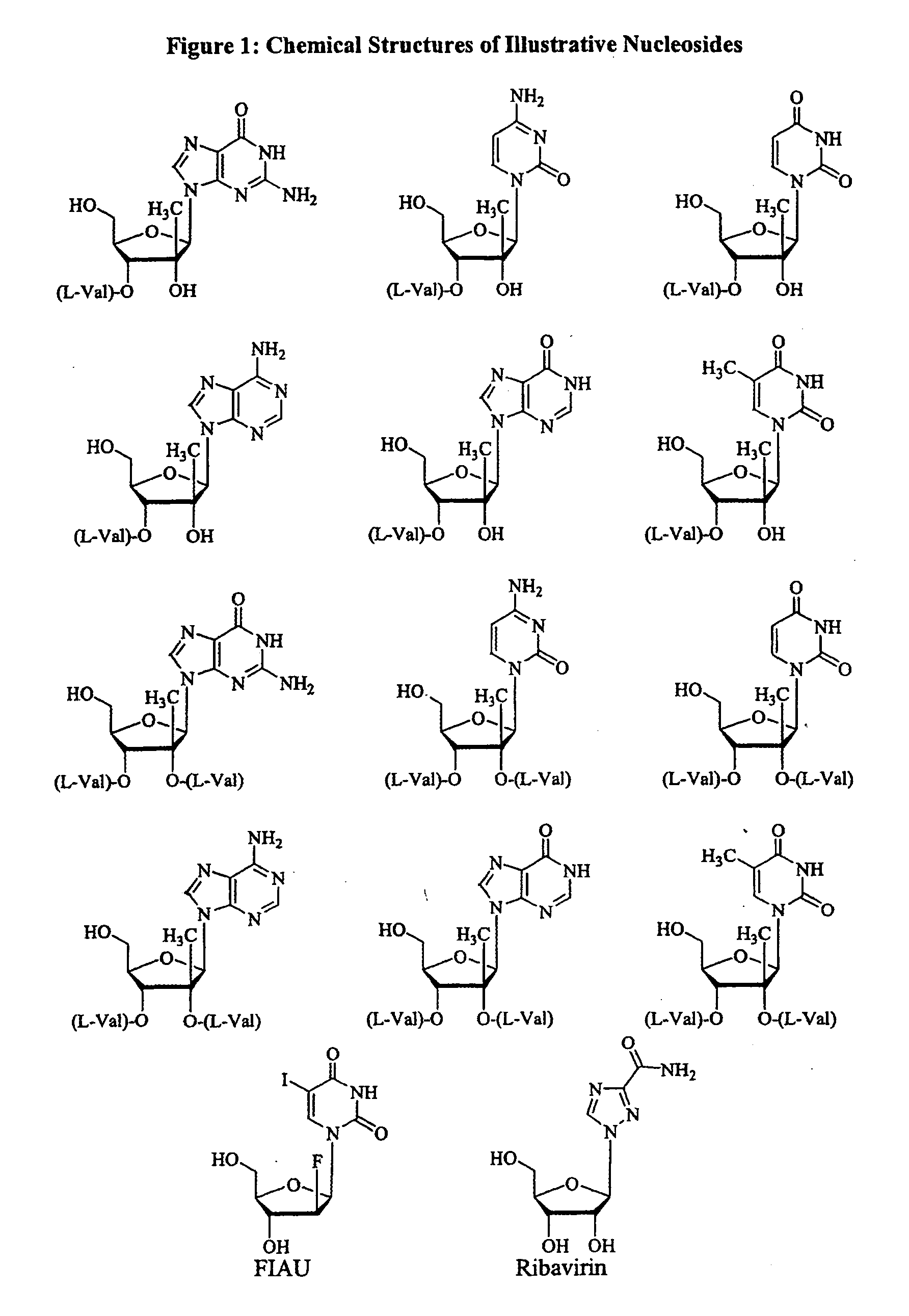
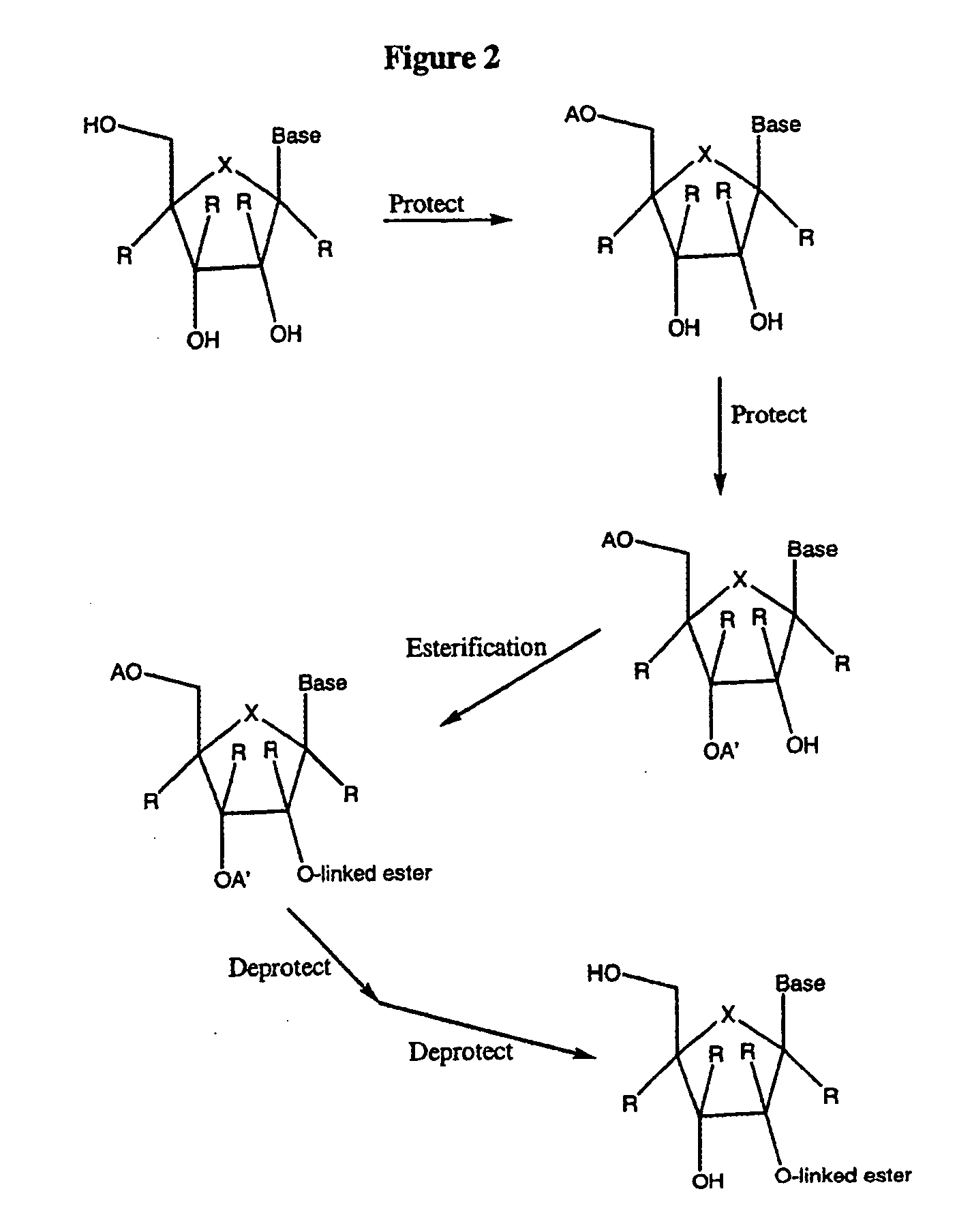
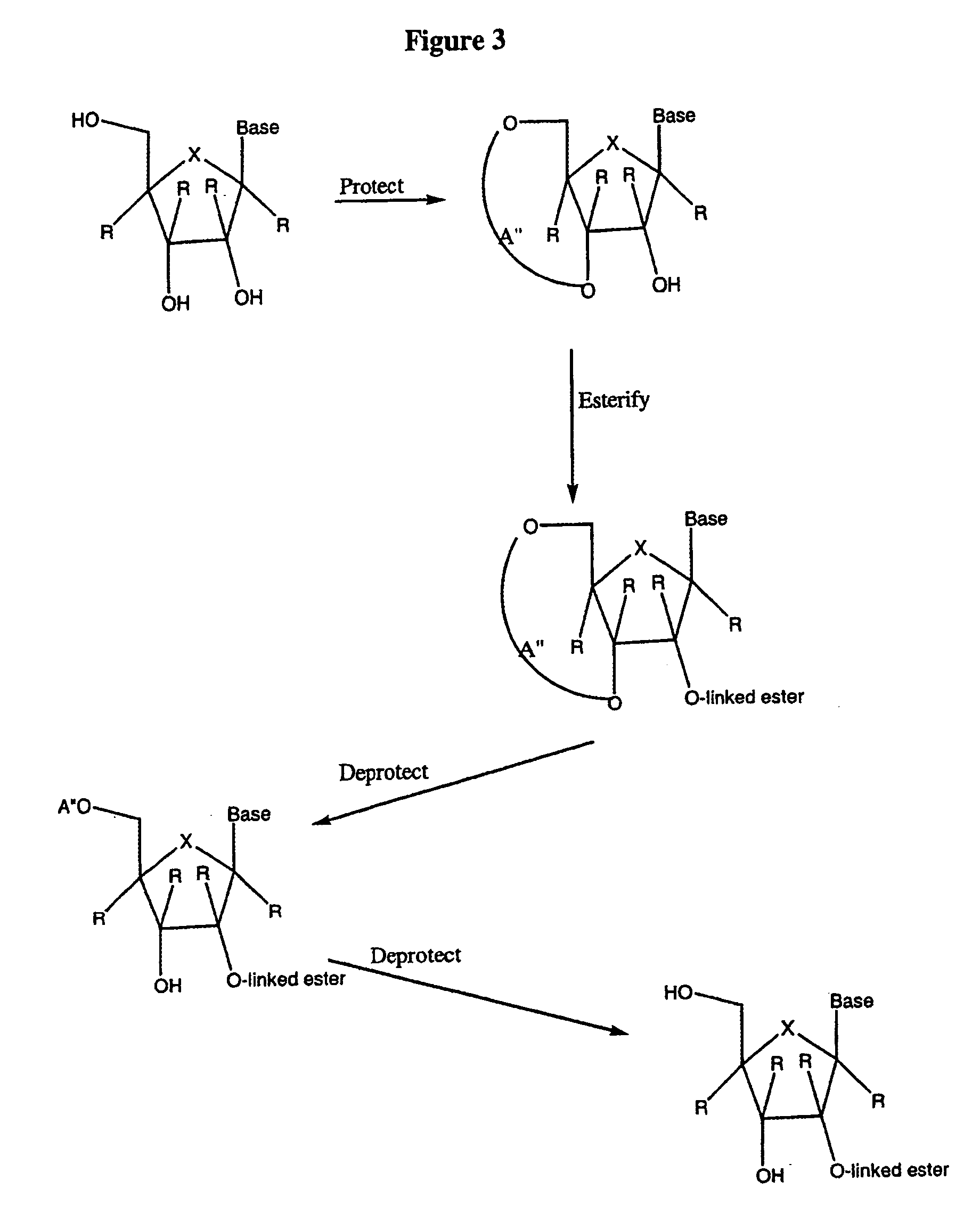
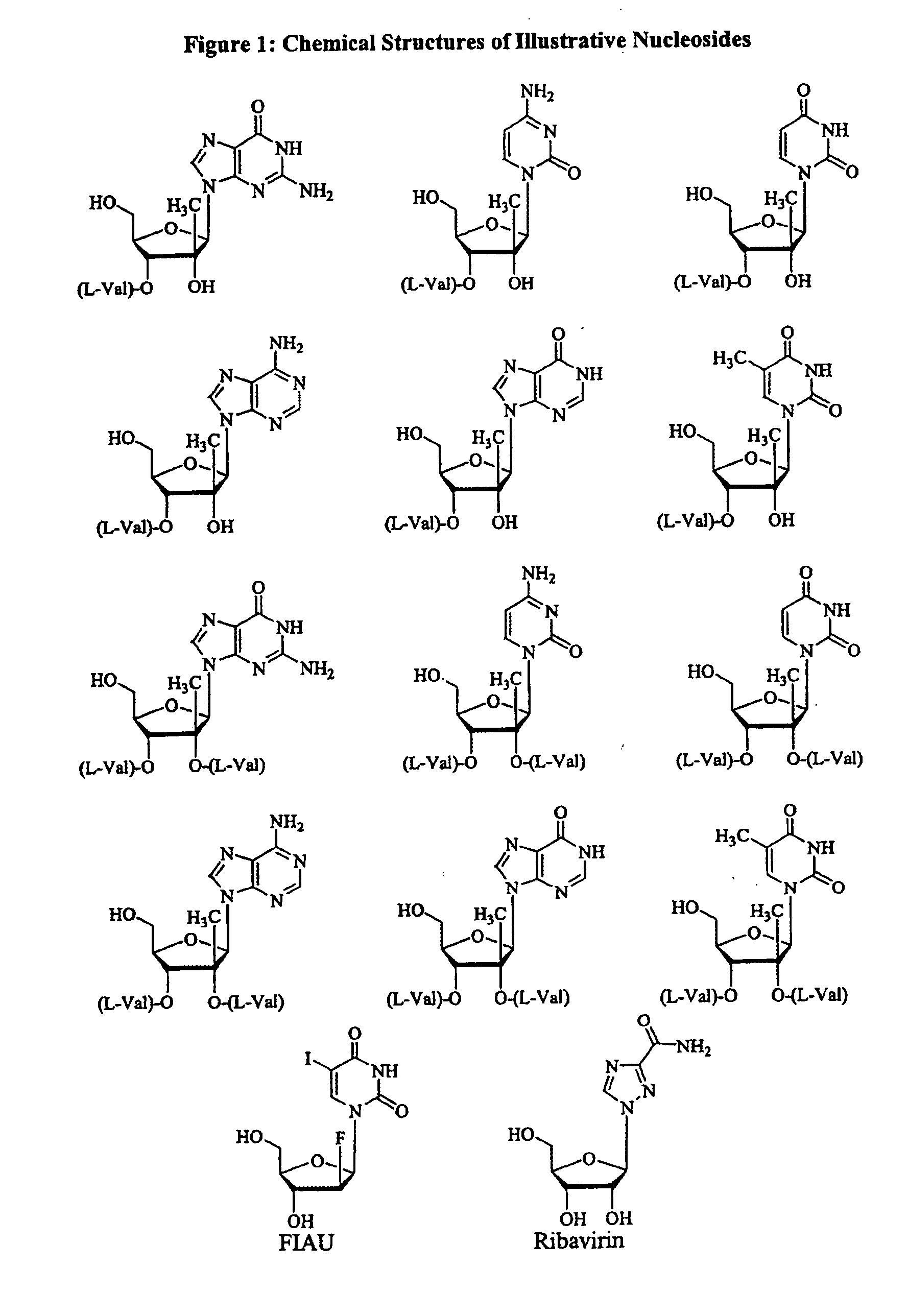
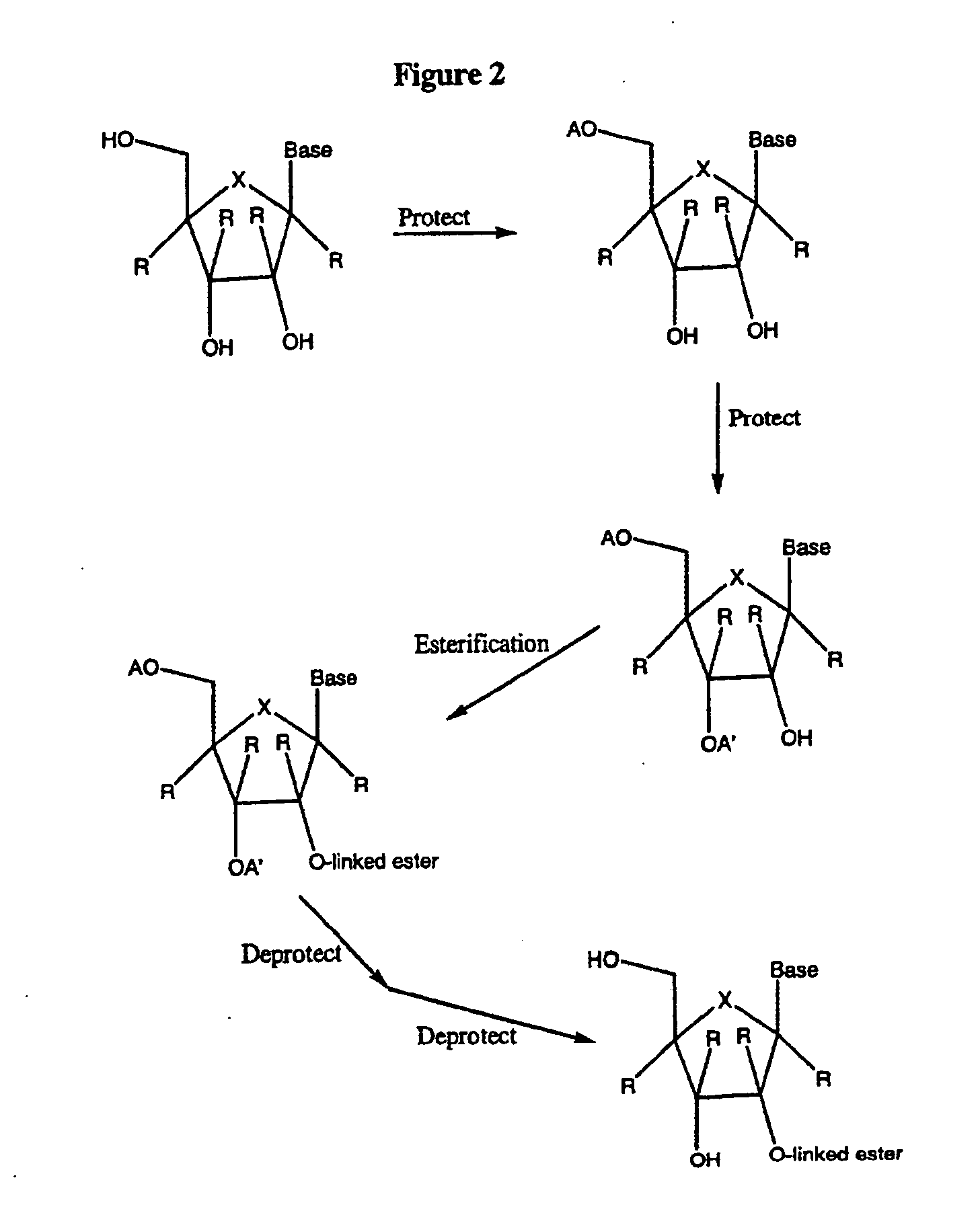
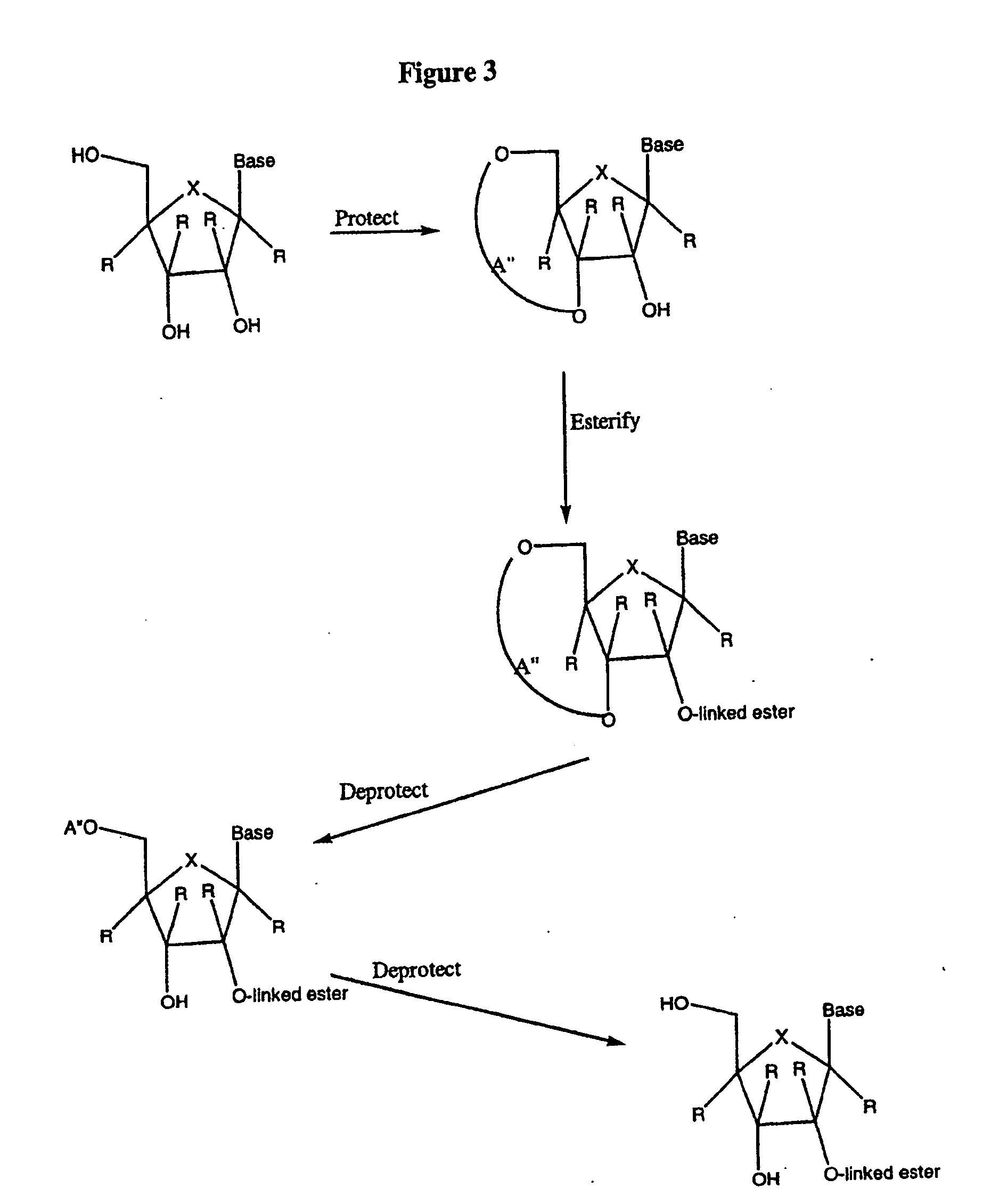
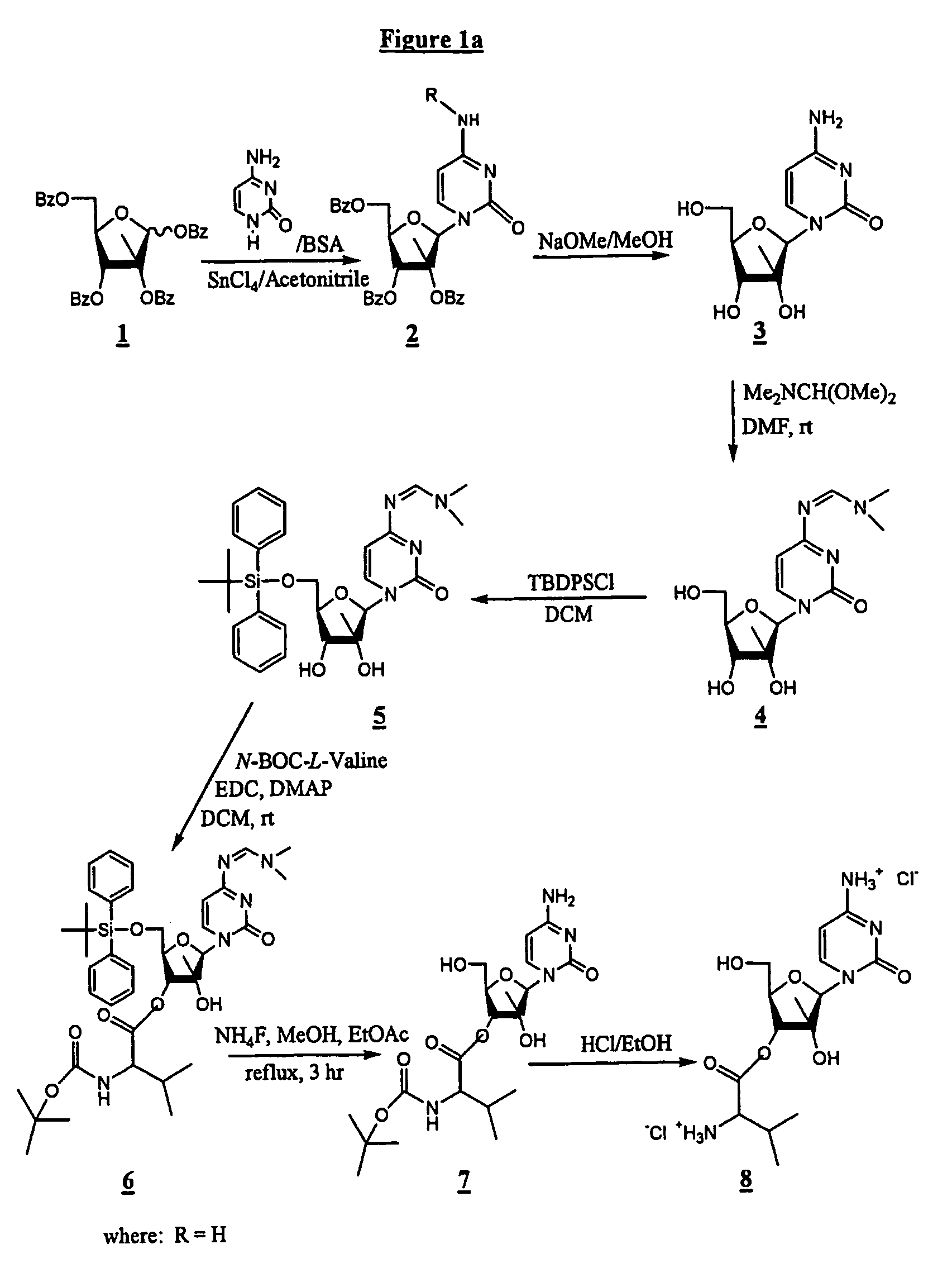
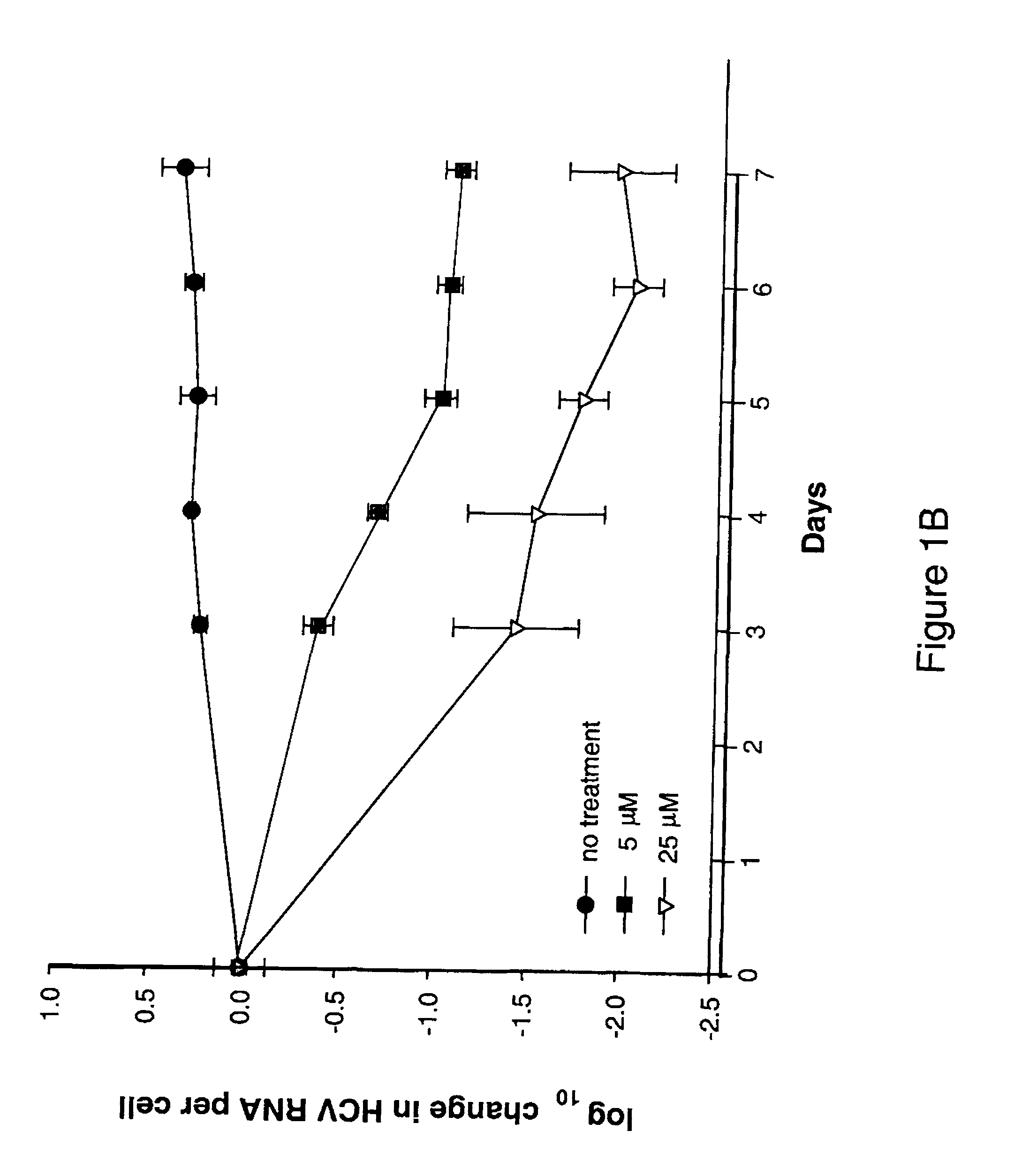
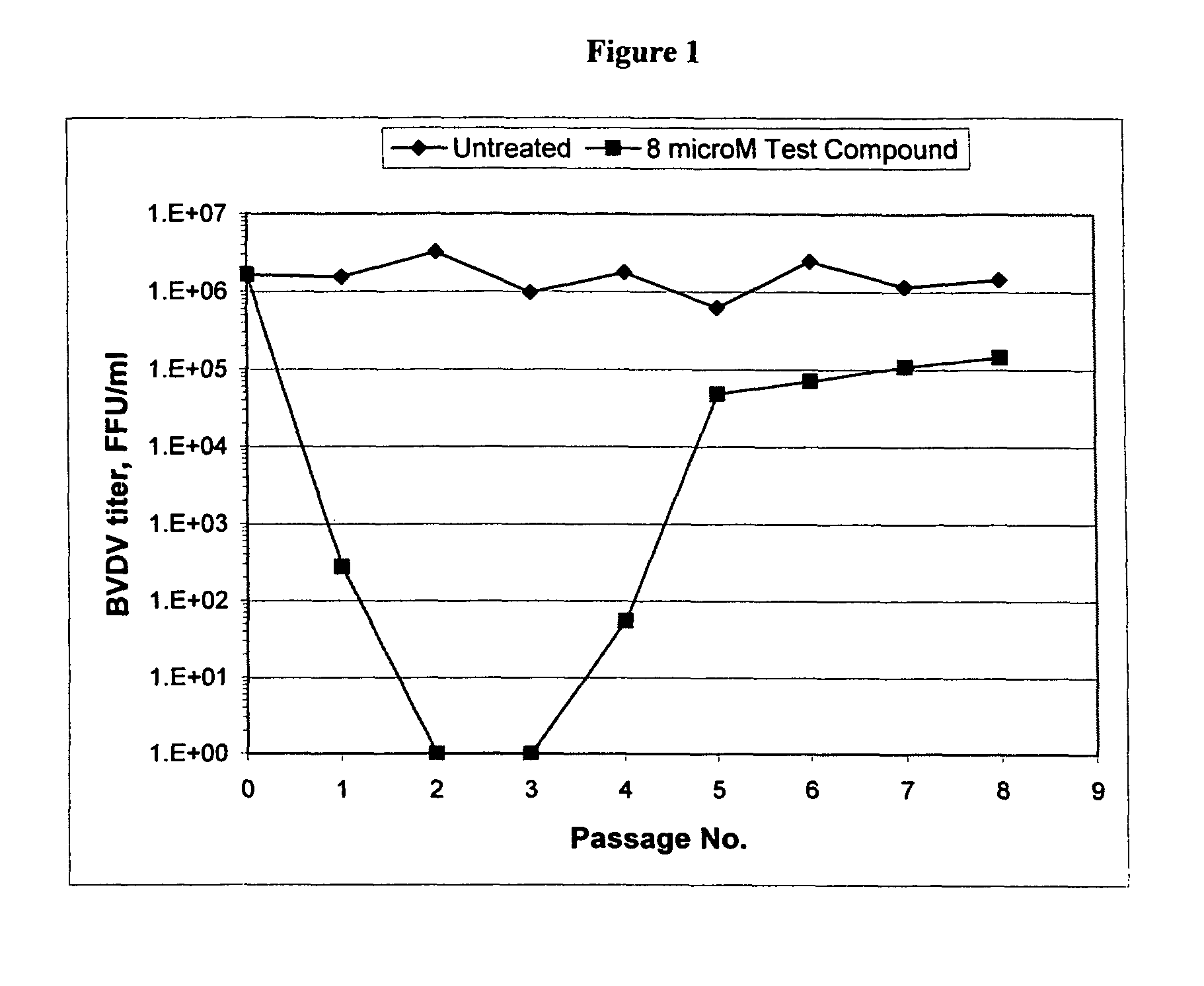
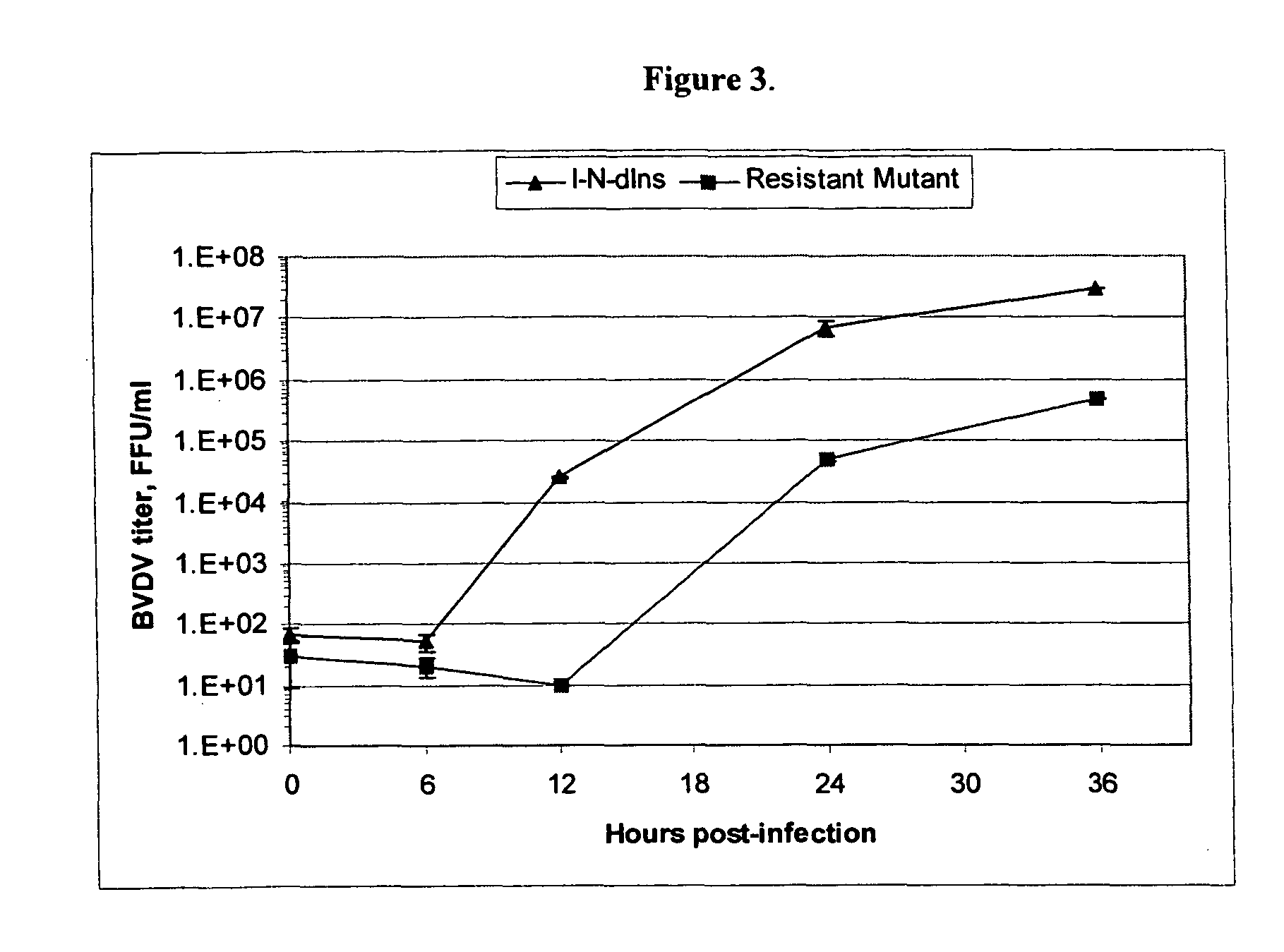
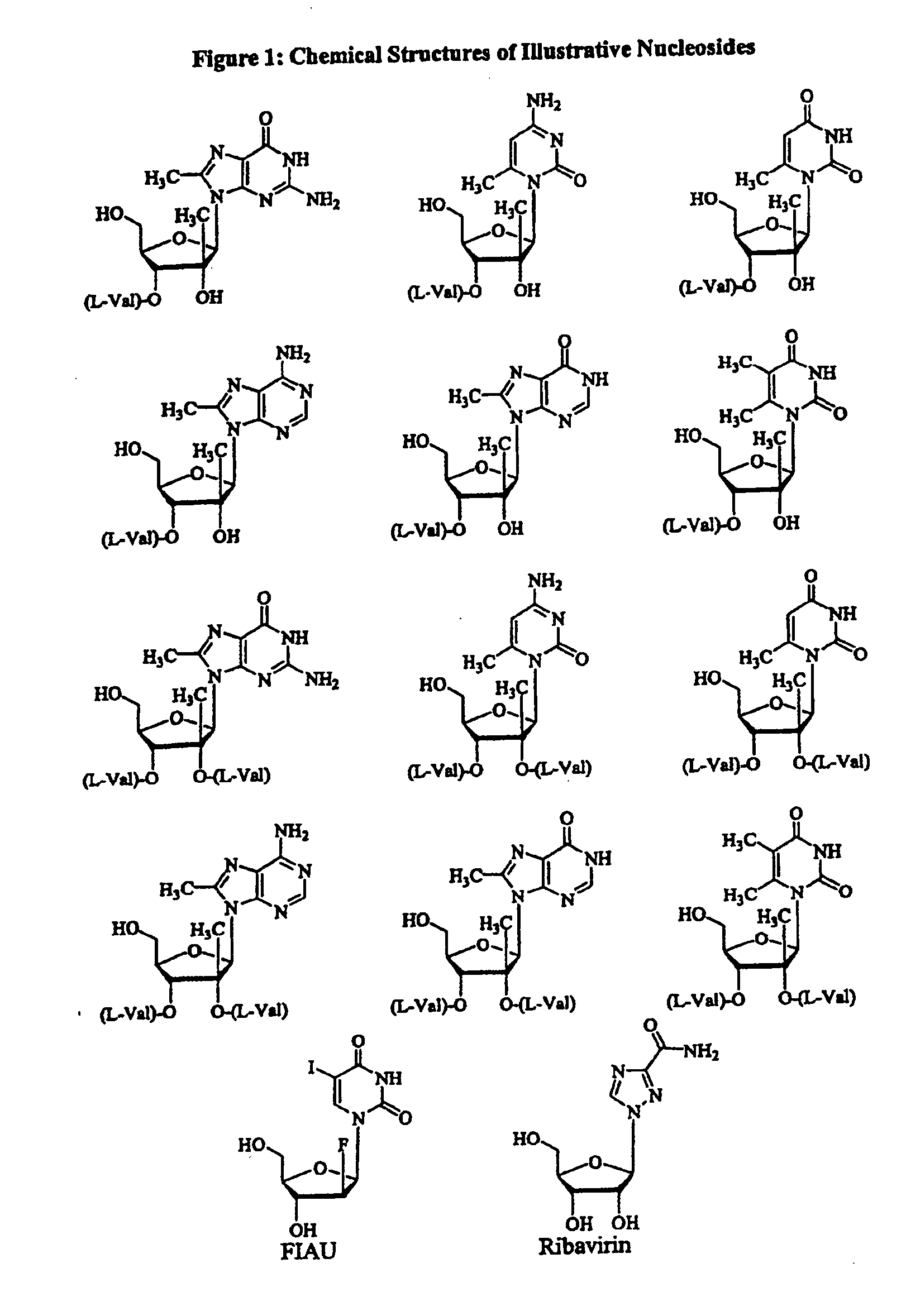
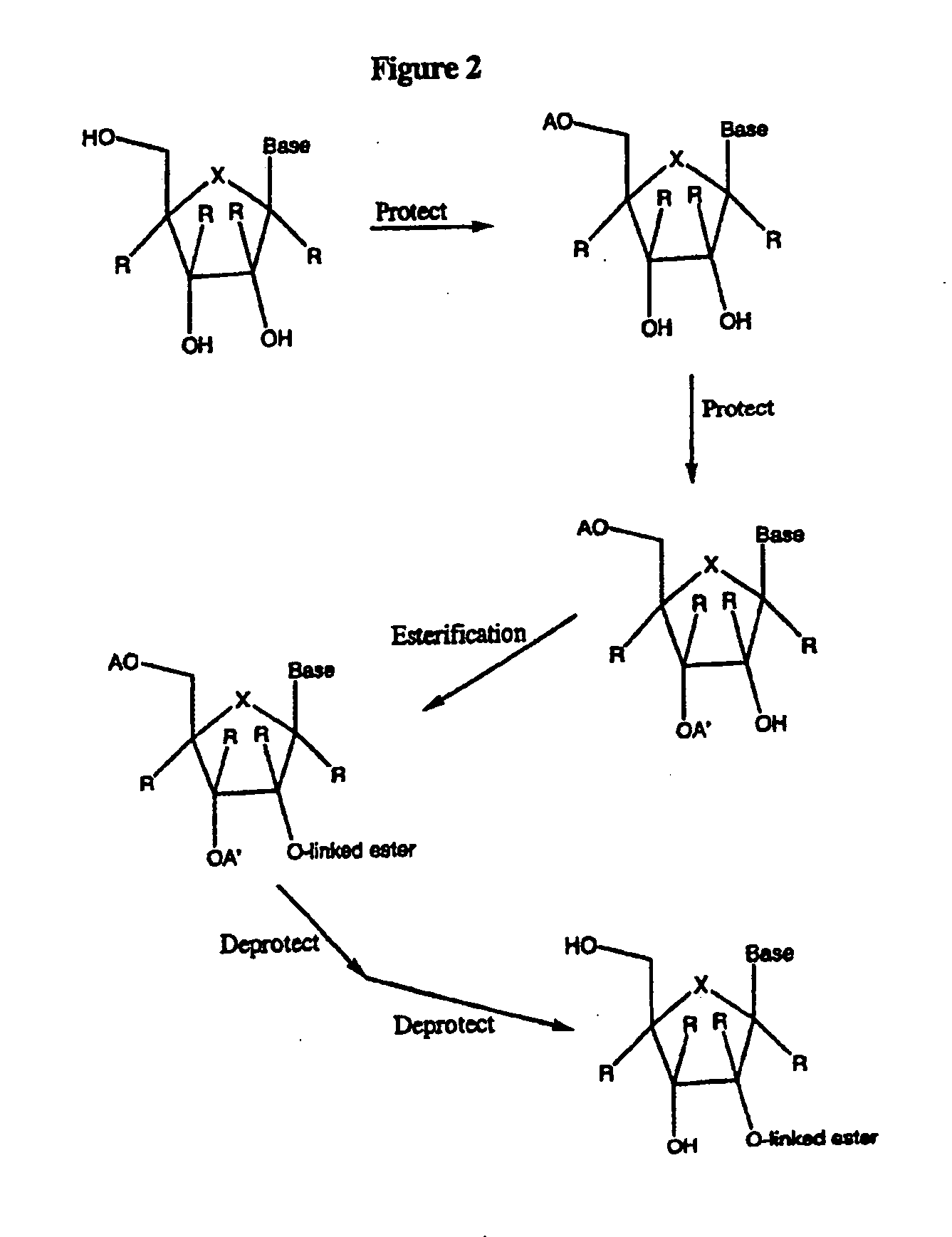
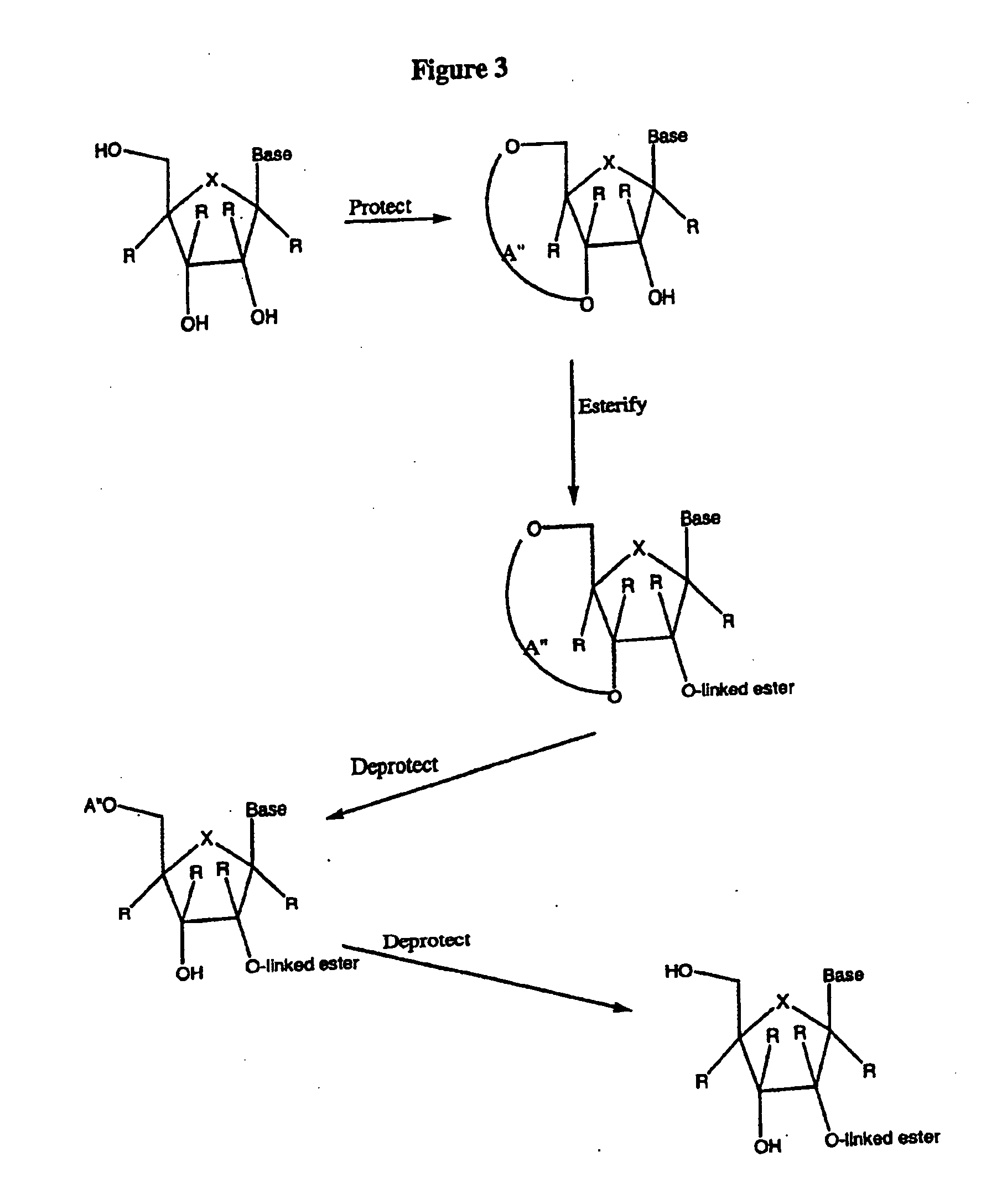
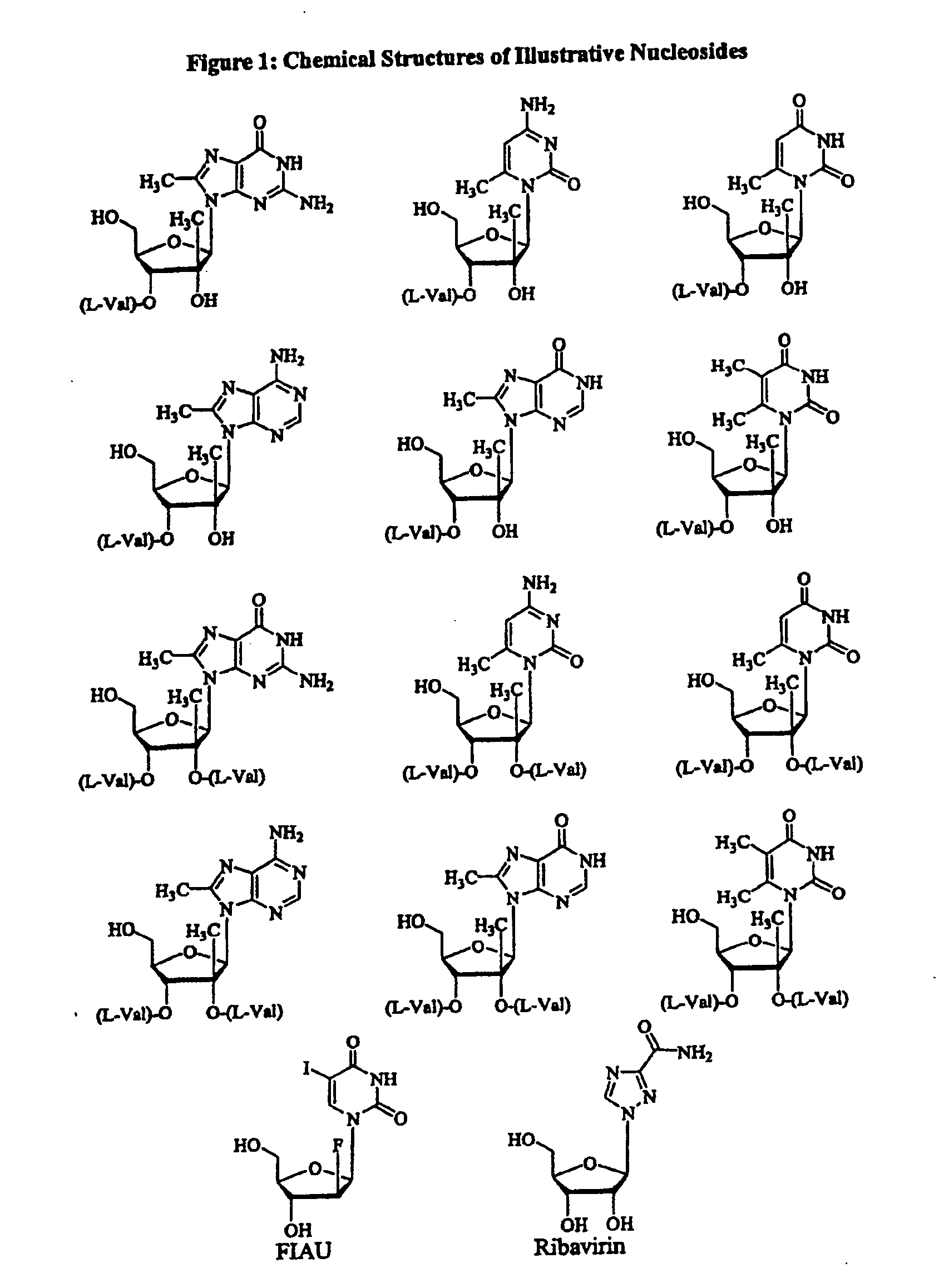
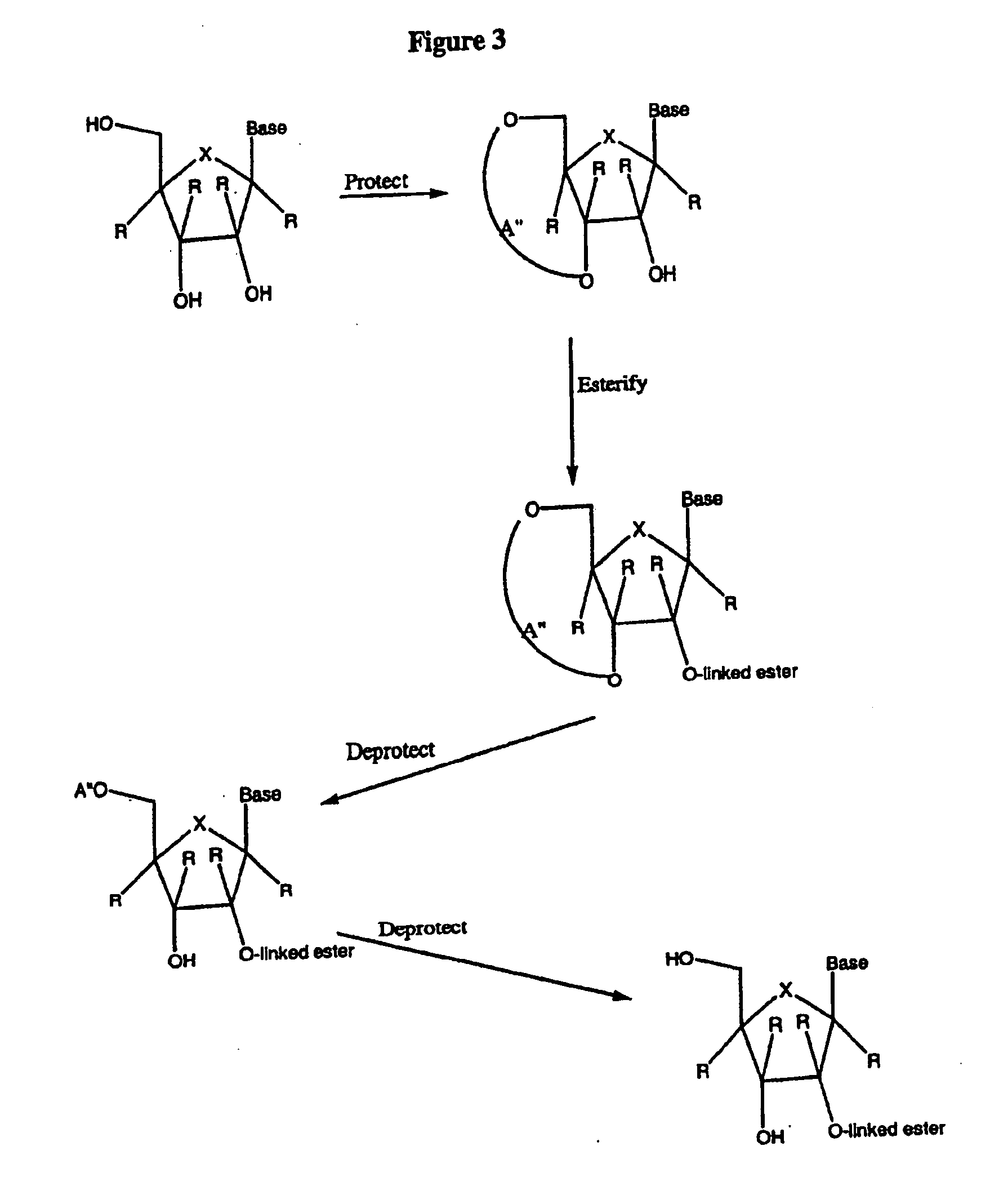
Spiro[2.4]heptanes for treatment of flaviviridae infections
Compounds, methods, and compositions for the treatment of infections in or exposure to humans and other host animals of Flaviviridae viruses, including HCV, that includes the administration of an effective amount of a spiro[2.4]heptane as described herein or a pharmaceutically acceptable salt or prodrug thereof, optionally in a pharmaceutically acceptable carrier, are provided. The spiro[2.4]heptane compounds either possess antiviral activity, or are metabolized to a compound that exhibits such activity.
Owner:UNIV OF GEORGIA RES FOUND INC
Andrographolide derivatives to treat viral infections
The present invention provides a methods and compositions for treating a host afflicted with a viral infection, particularly a Flaviviridae infection, including hepatitis C infection, comprising administering an effective antiviral amount of a derivative of andrographolide alone or in combination or alternation with another antiviral compound.
Owner:CORNELL RES FOUNDATION INC
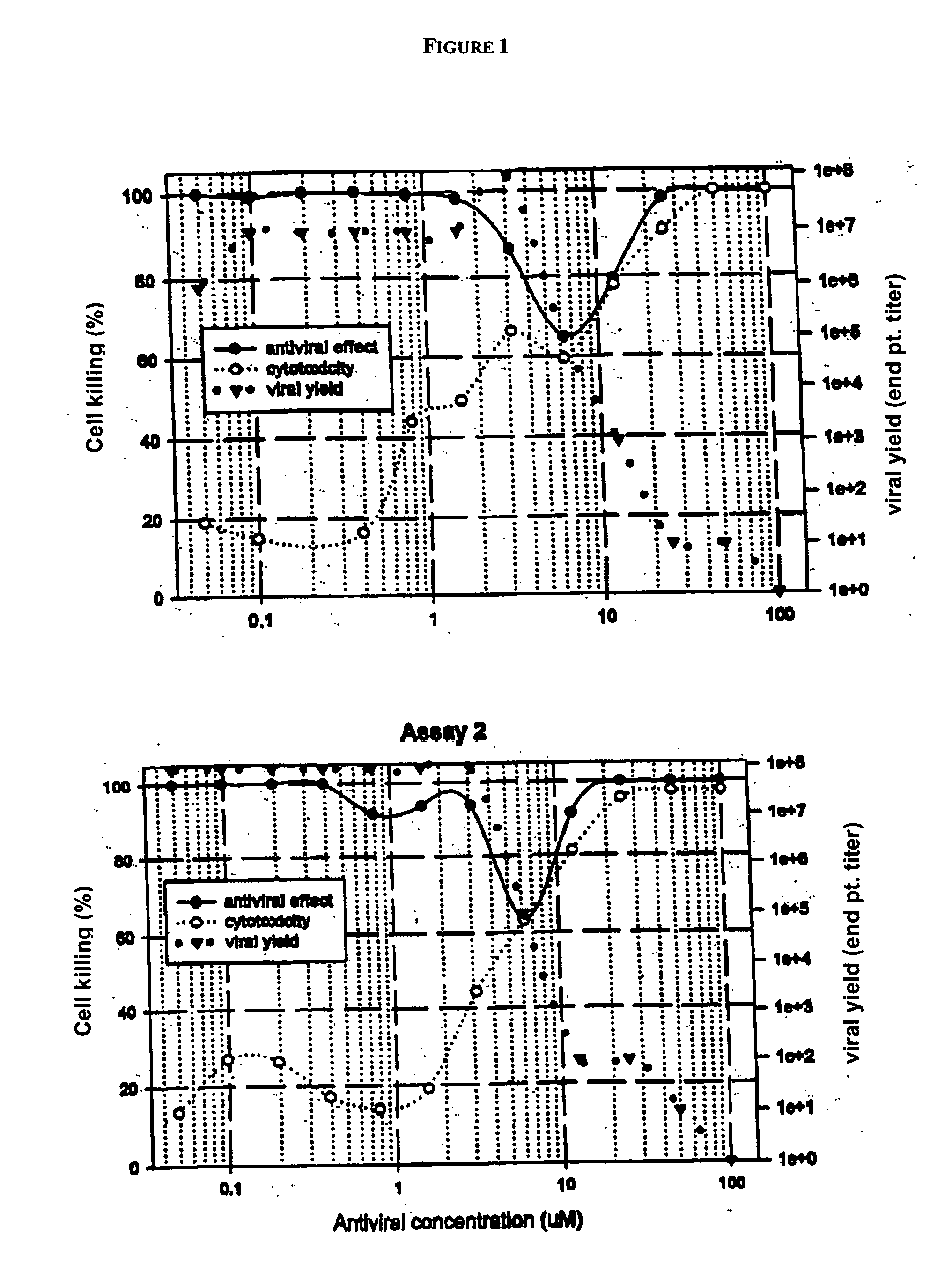
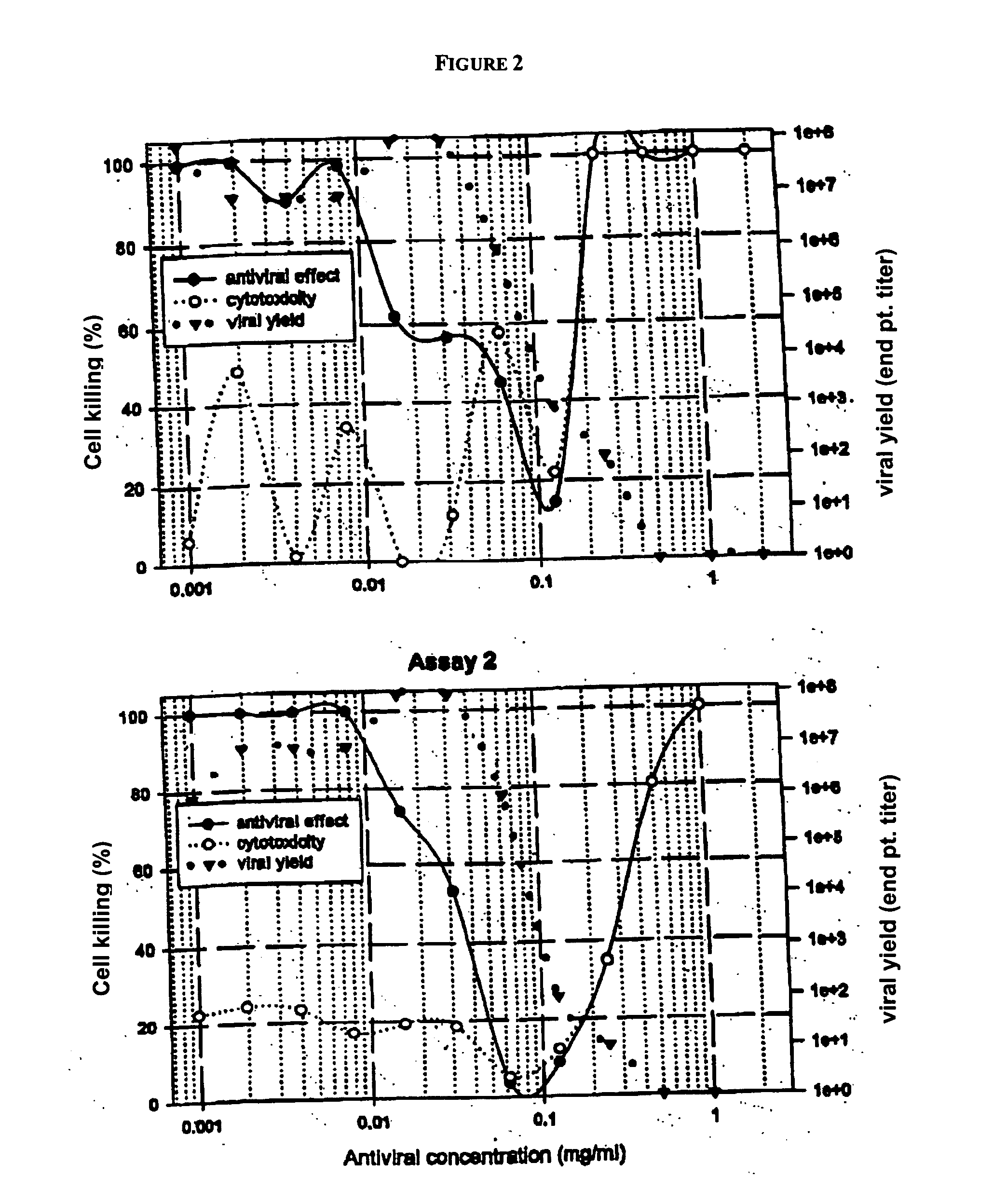
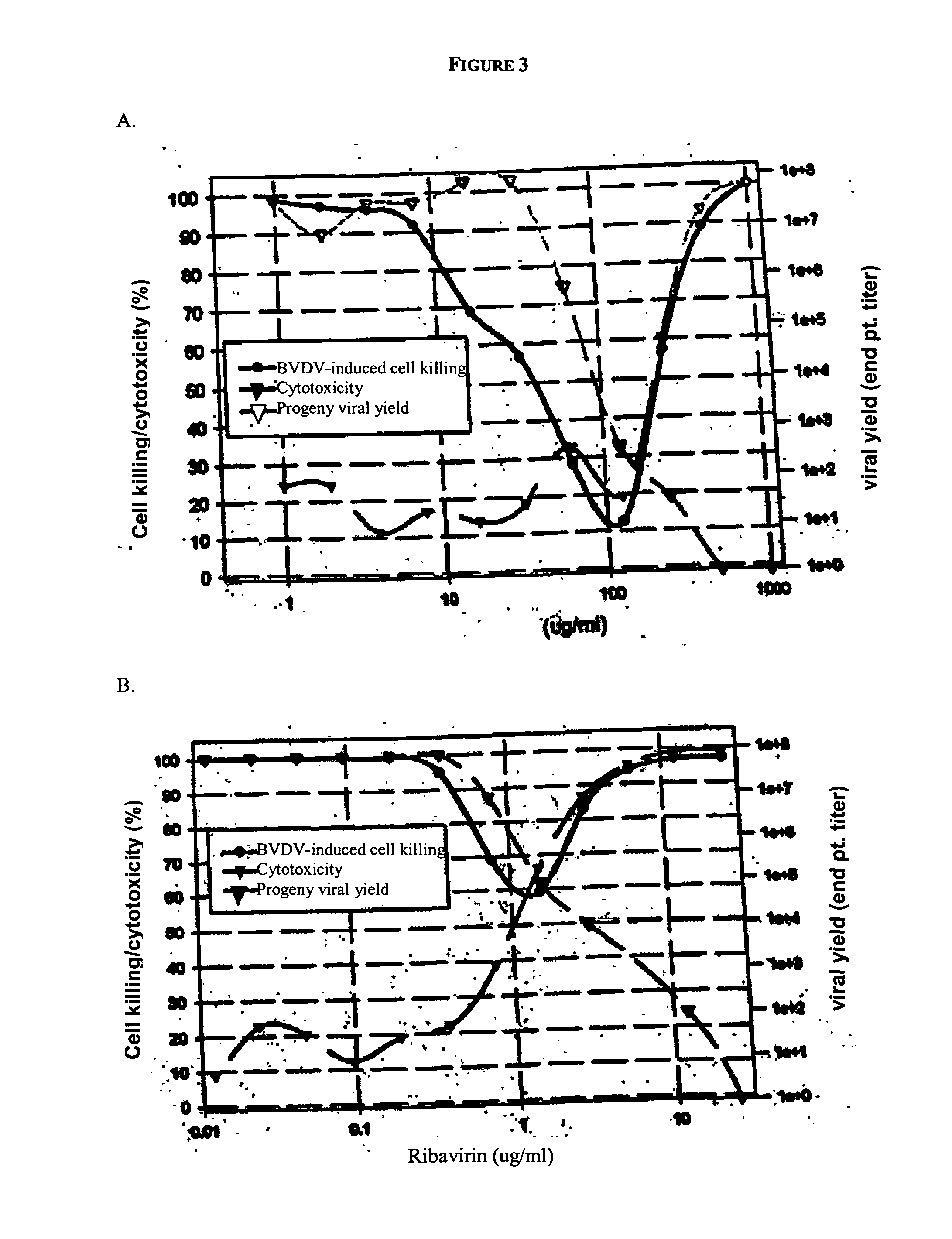
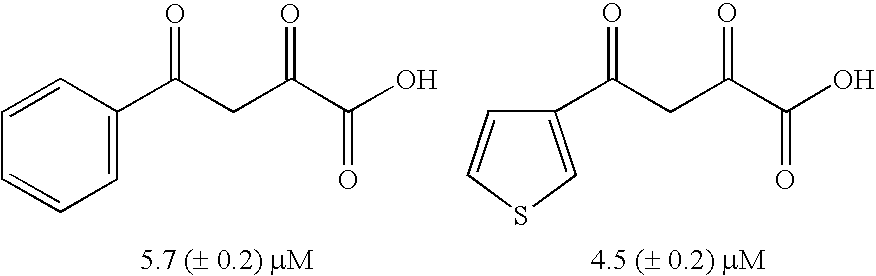
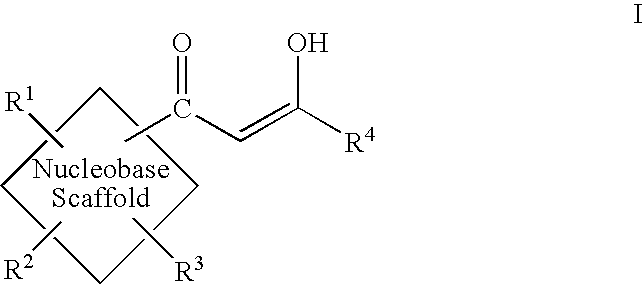
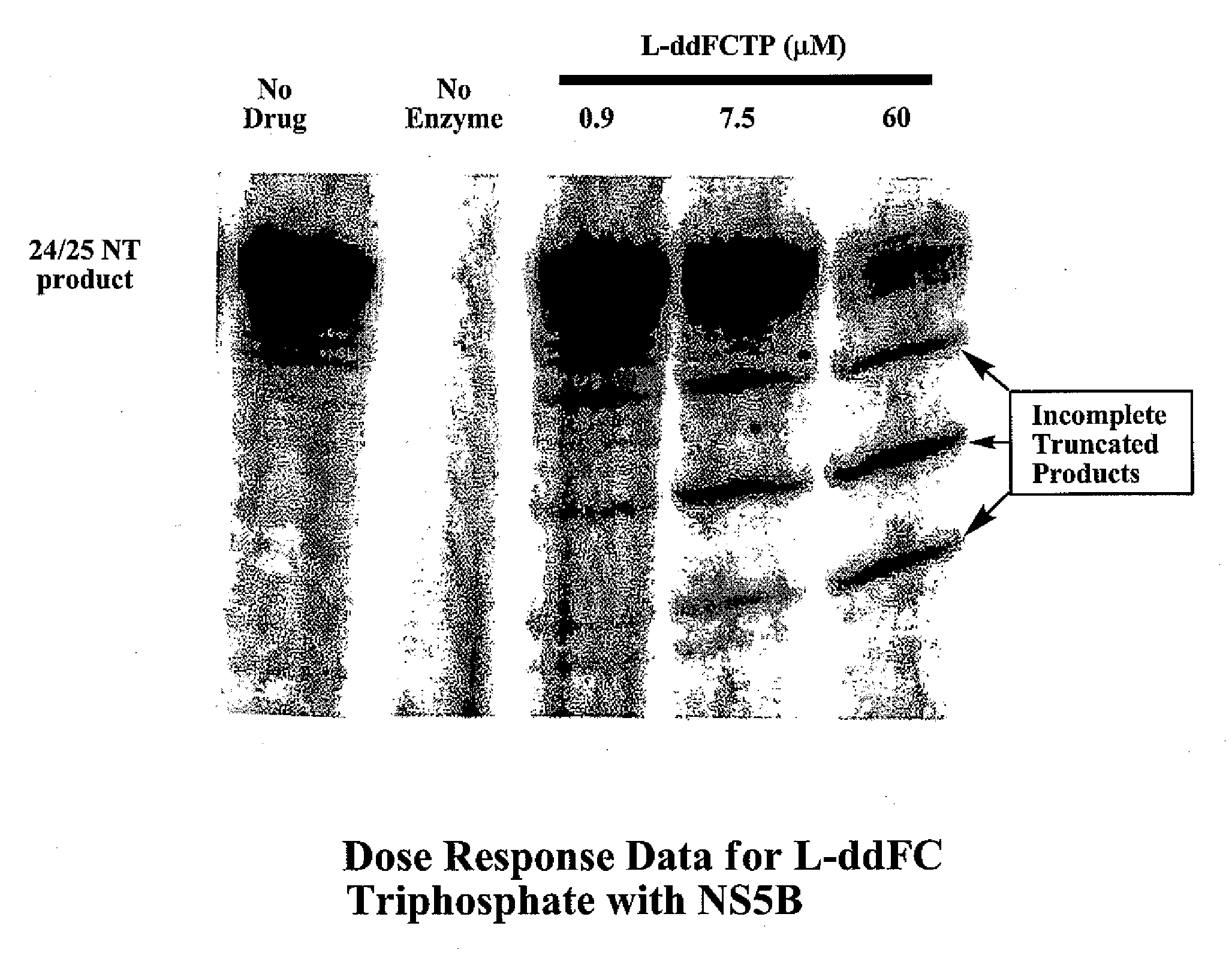
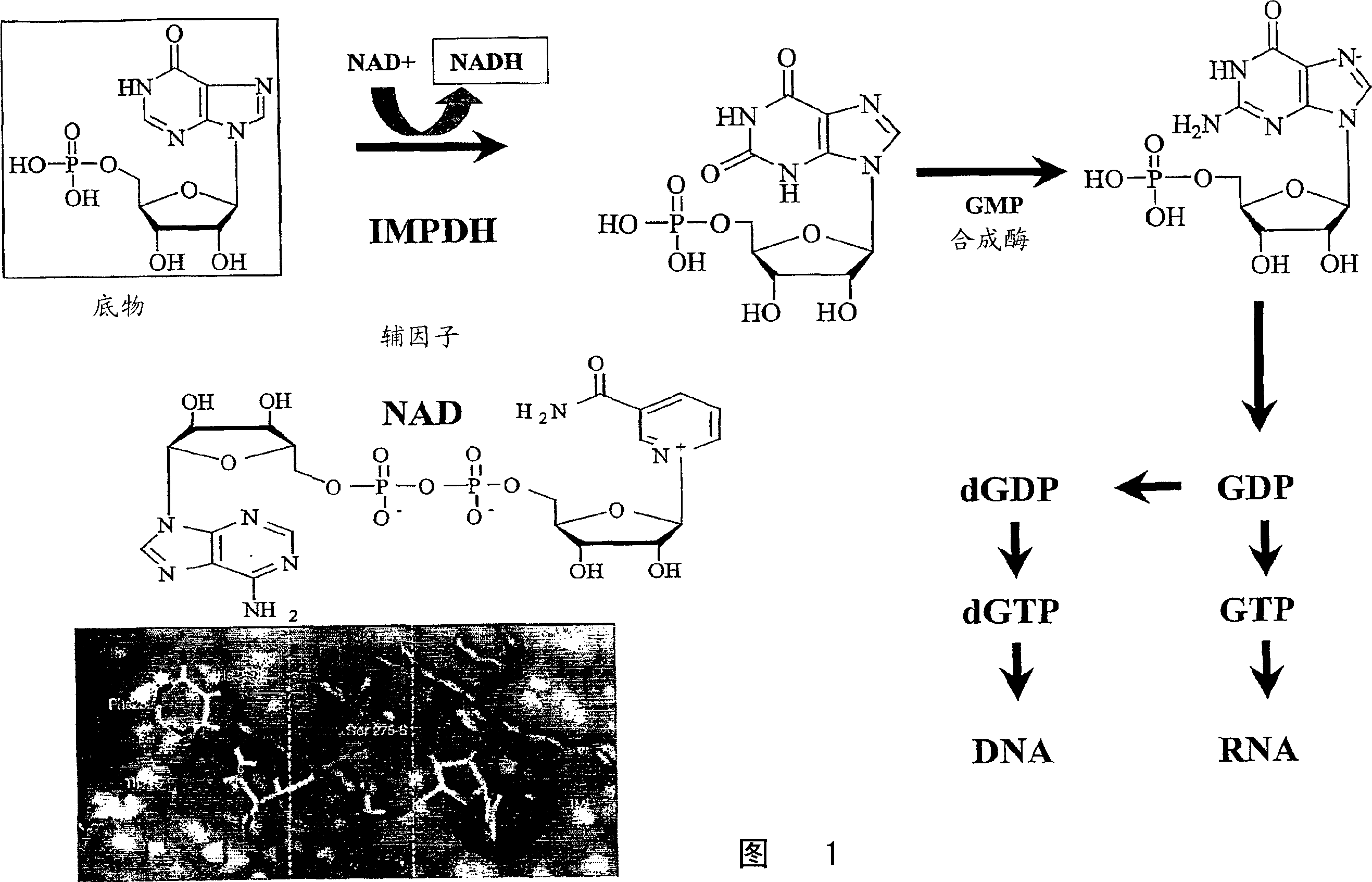
Diketo acids on nucleobase scaffolds as inhibitors of Flaviviridae
A new class of diketo acids constructed on nucleobase scaffolds, designed as inhibitors of HCV replication through inhibition of HCV NS5B RNA polymerase, is described. These compounds are useful in the prevention or treatment of infection by HCV and in the treatment of other Flaviviridae infections, either as the compounds, or as pharmaceutically acceptable salts, with pharmaceutically acceptable carriers, used alone or in combination with antivirals, immunomodulators, antibiotics, vaccines, and other therapeutic agents. Methods of treating HCV and methods of treating or preventing infection by HCV are also described.
Owner:UNIV OF GEORGIA RES FOUND INC
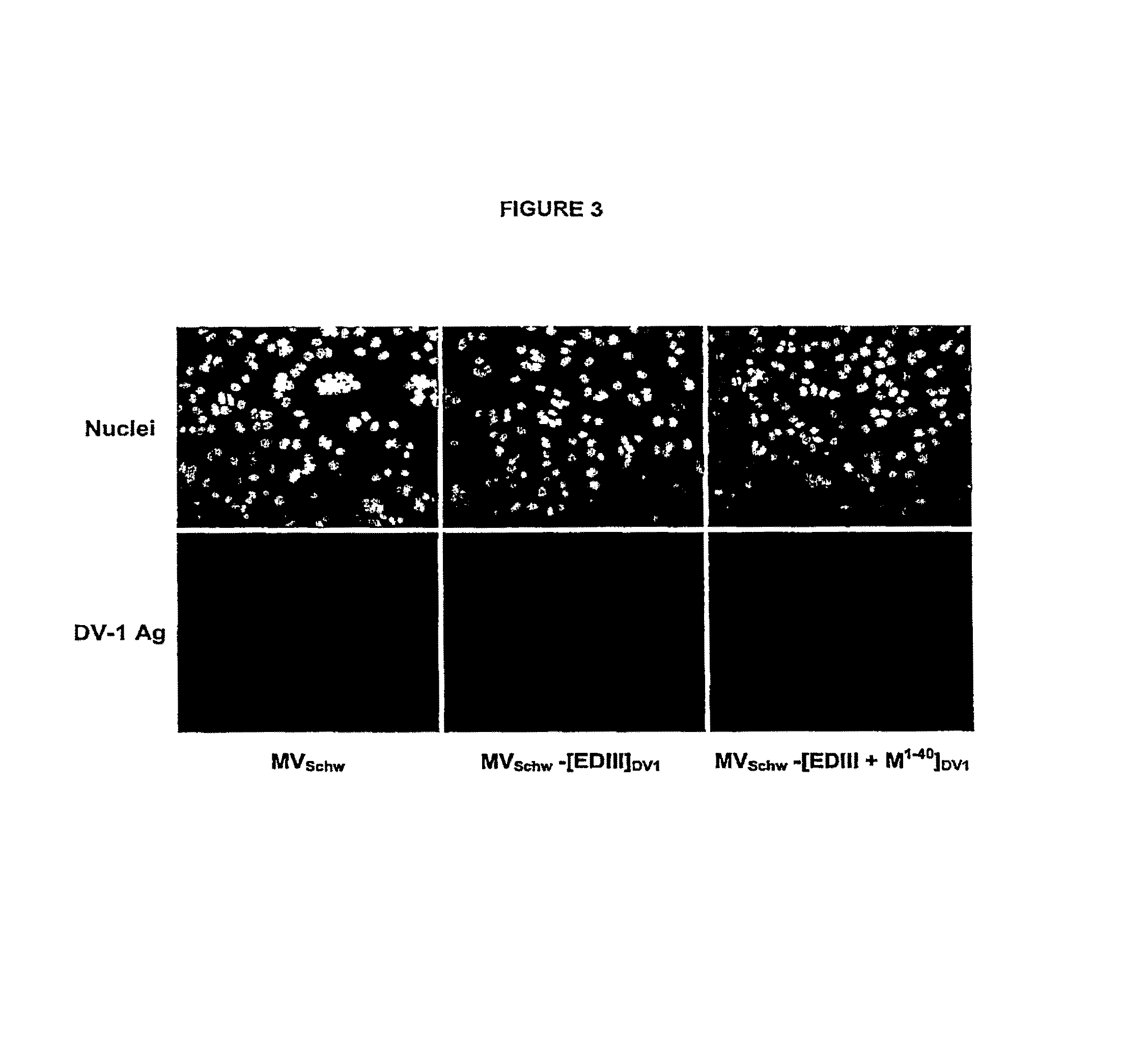
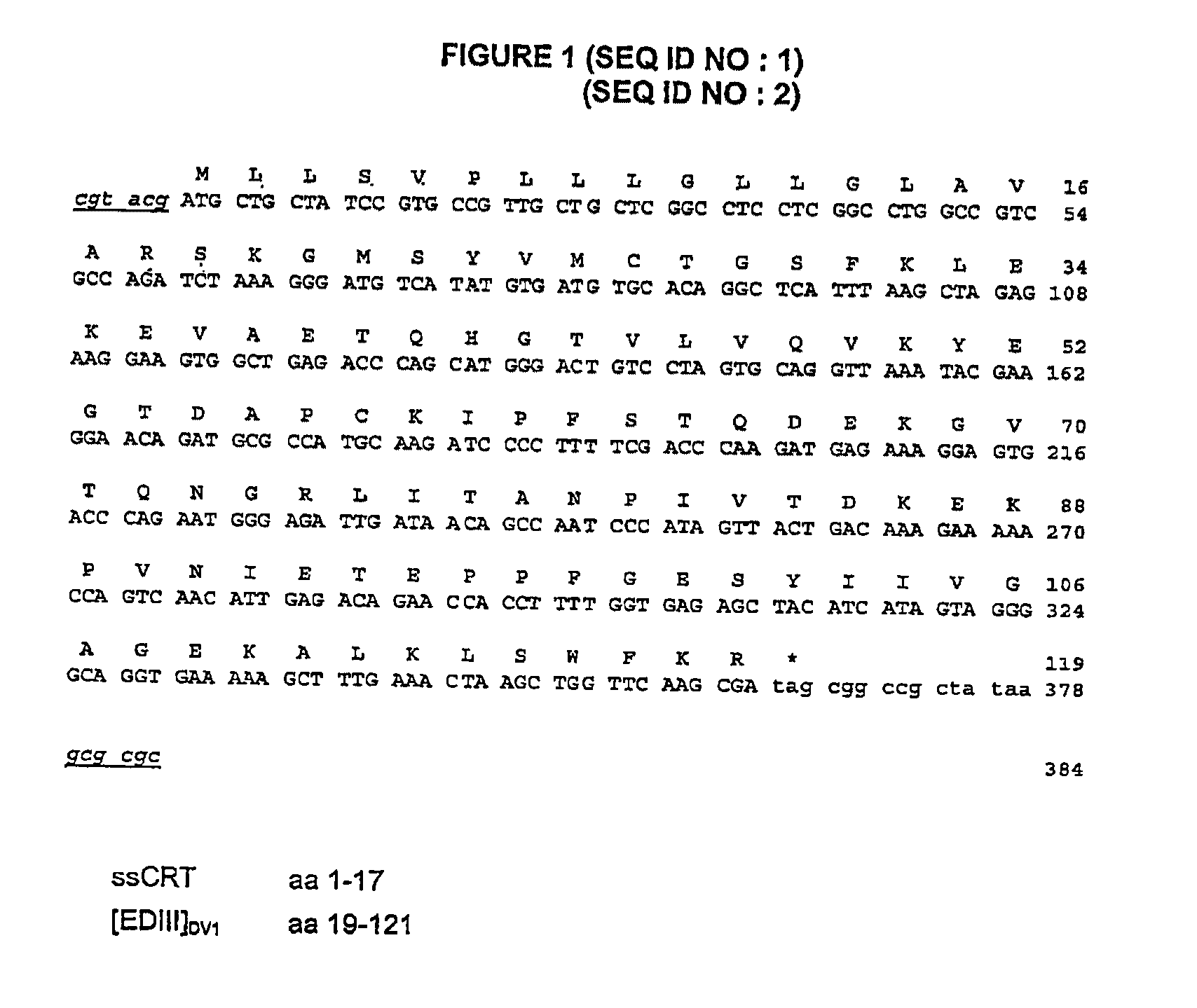
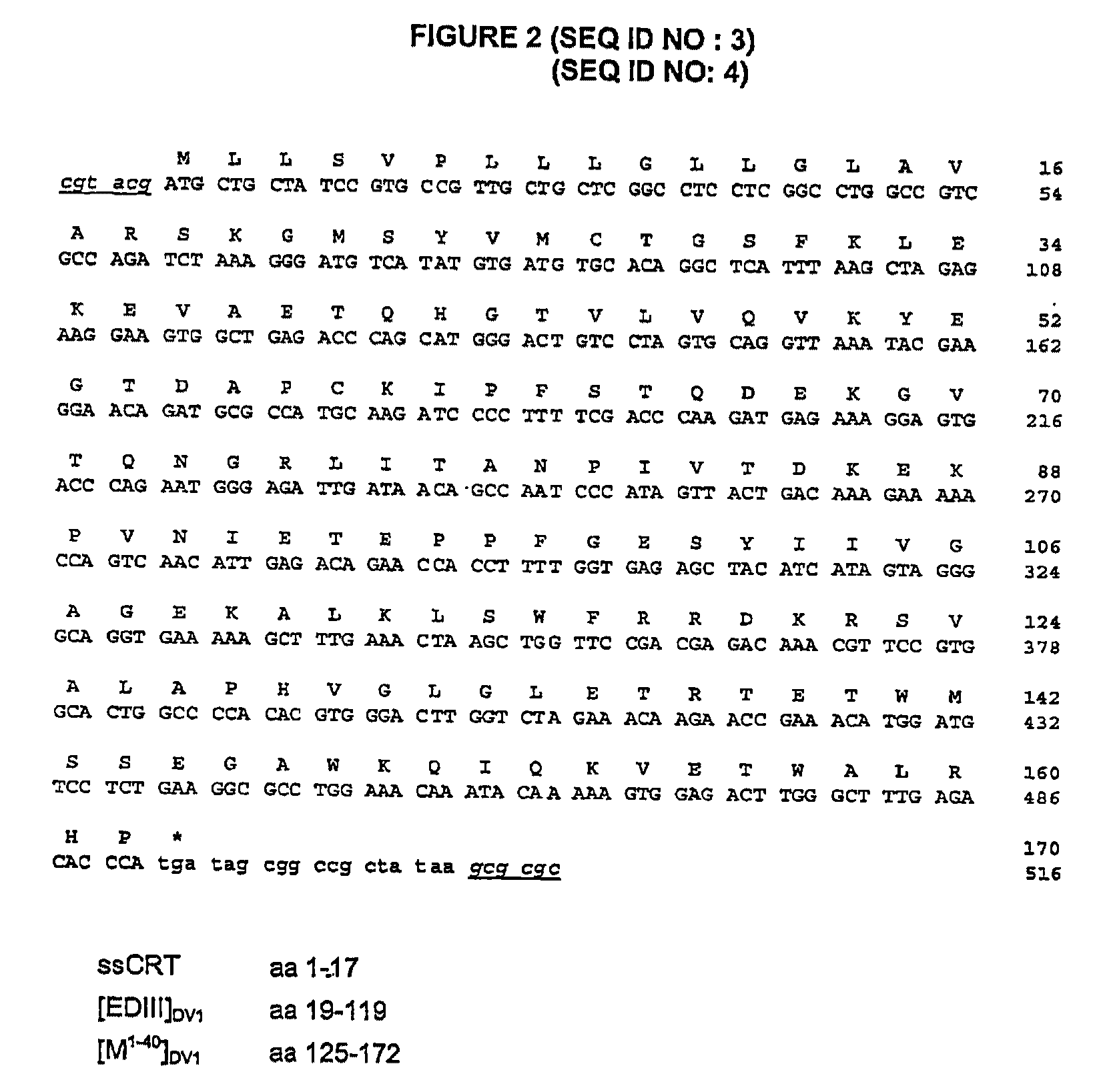
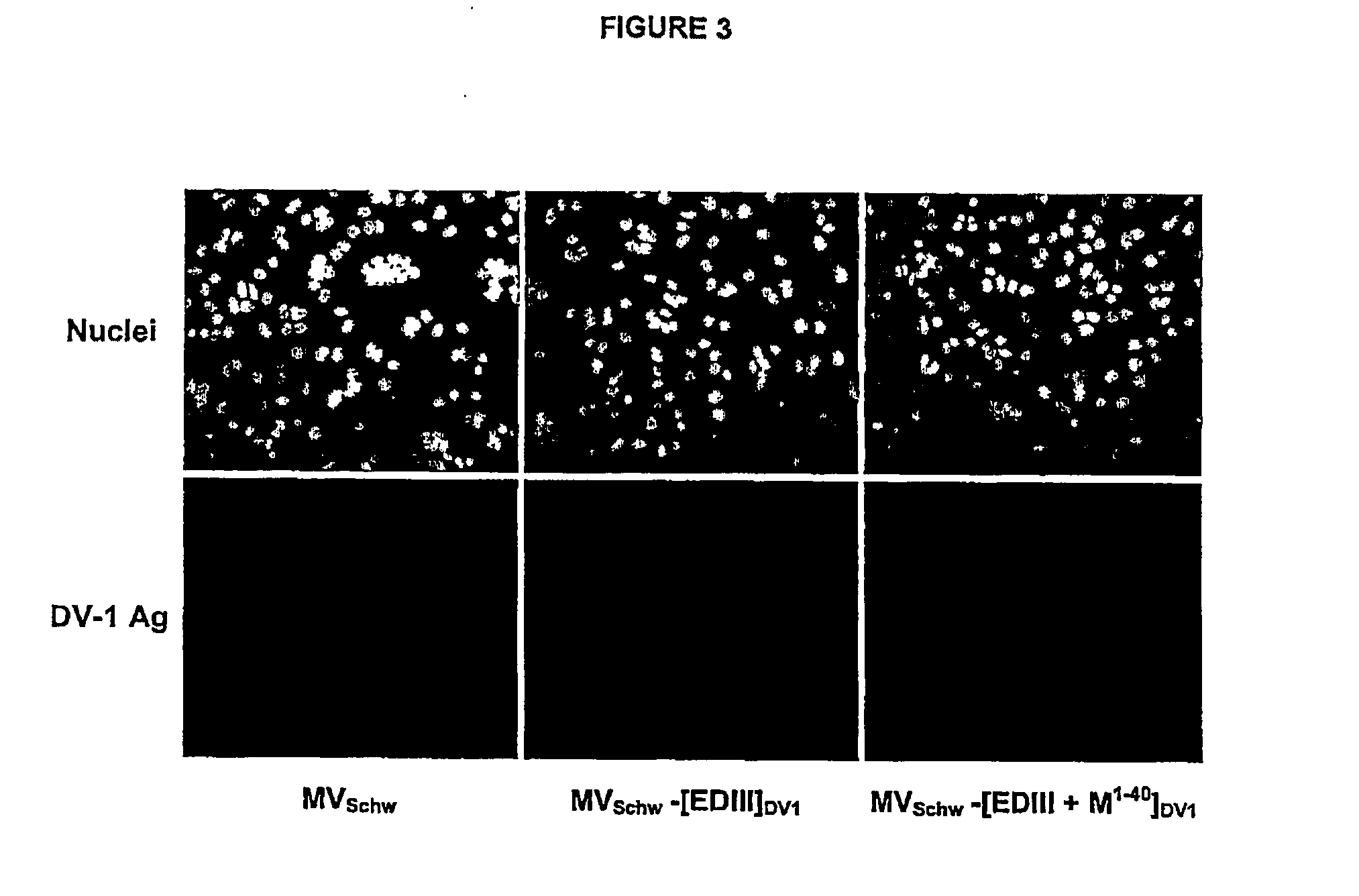
Chimeric poly peptides and the therapeutic use thereof against a Flaviviridae infection
The invention relates to building a chimeric polypeptide used for preventing or treating a Flaviviridae infection. The use of the inventive chimeric polypeptide for producing recombinant viral vectors such as a measles living viral vector is also disclosed.
Owner:INST PASTEUR +1
Lysine demethylase inhibitors for diseases and disorders associated with Flaviviridae
ActiveUS9790196B2Avoid side effectsReduce duplicationOrganic active ingredientsBiocideLysineLuetic disease
The invention relates to methods and compositions for the treatment or prevention of Flaviviridae infections. In particular, the invention relates to an LSD1 inhibitor for use in treating or preventing Flaviviridae infections, including hepatitis C virus infections.
Owner:ORYZON GENOMICS SA
Modified 2' and 3' -nucleoside produgs for treating flaviridae infections
2' and / or 3'-prodrugs of 1', 2', 3' or 4'-branched nucleosides, and their pharmaceutically acceptable salts and derivatives are described. These prodrugs are used in the prophylaxis and treatment of Flaviviridae infections, including HCV infections, and other related conditions. Compounds and compositions of the prodrugs of the invention are described. Also provided are methods and uses comprising administering an effective amount of a prodrug of the invention, or a pharmaceutically acceptable salt or derivative thereof. These agents may optionally be administered in combination or alternately with other antiviral agents to prevent or treat flavivirus infection and other related conditions.
Owner:INDENIX PHARM LLC +3
Compounds for the treatment of flaviviridae infections
InactiveUS20050049204A1Lower Level RequirementsLower levelBiocideDigestive systemBovine Viral Diarrhea VirusesL-Aspartate
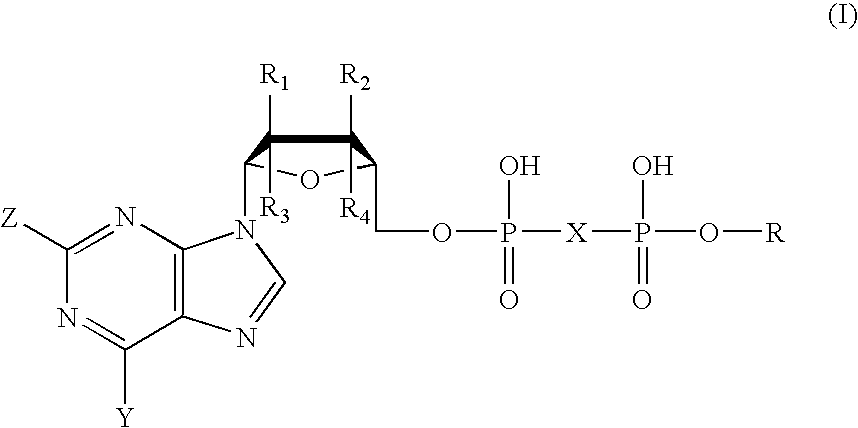
The disclosed invention is a composition for and a method of treating a Flaviviridae infections, such as bovine viral diarrhea virus (“BVDV”), Dengue Virus (DENV), West Nile Virus (WNV) and hepatitis C virus (HCV), as well as abnormal cellular proliferation, in a host, including animals, and especially humans, using a nucleoside of general formula (I)-(V) or N-(phosphonoacetyl)-L-aspartate (PALA), or a pharmaceutically acceptable salt or prodrug thereof.
Owner:PHARMASSET
Modified 2' and 3'-nucleoside prodrugs for treating Flaviviridae infections
InactiveUS20070027066A1Prevent and retard progressionDetermine the spectrum of activityBiocideSaccharide peptide ingredientsNucleoside XProdrug
2′ and / or 3′ prodrugs of 1′, 2′, 3′ or 4′-branched nucleosides, and their pharmaceutically acceptable salts and derivatives are described. These prodrugs are useful in the prevention and treatment of Flaviviridae infections, including HCV infection, and other related conditions. Compounds and compositions of the prodrugs of the present invention are described. Methods and uses are also provided that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:THE CENT NAT DEL LA RECH SCIQUE +3
Compositions for HCV treatment
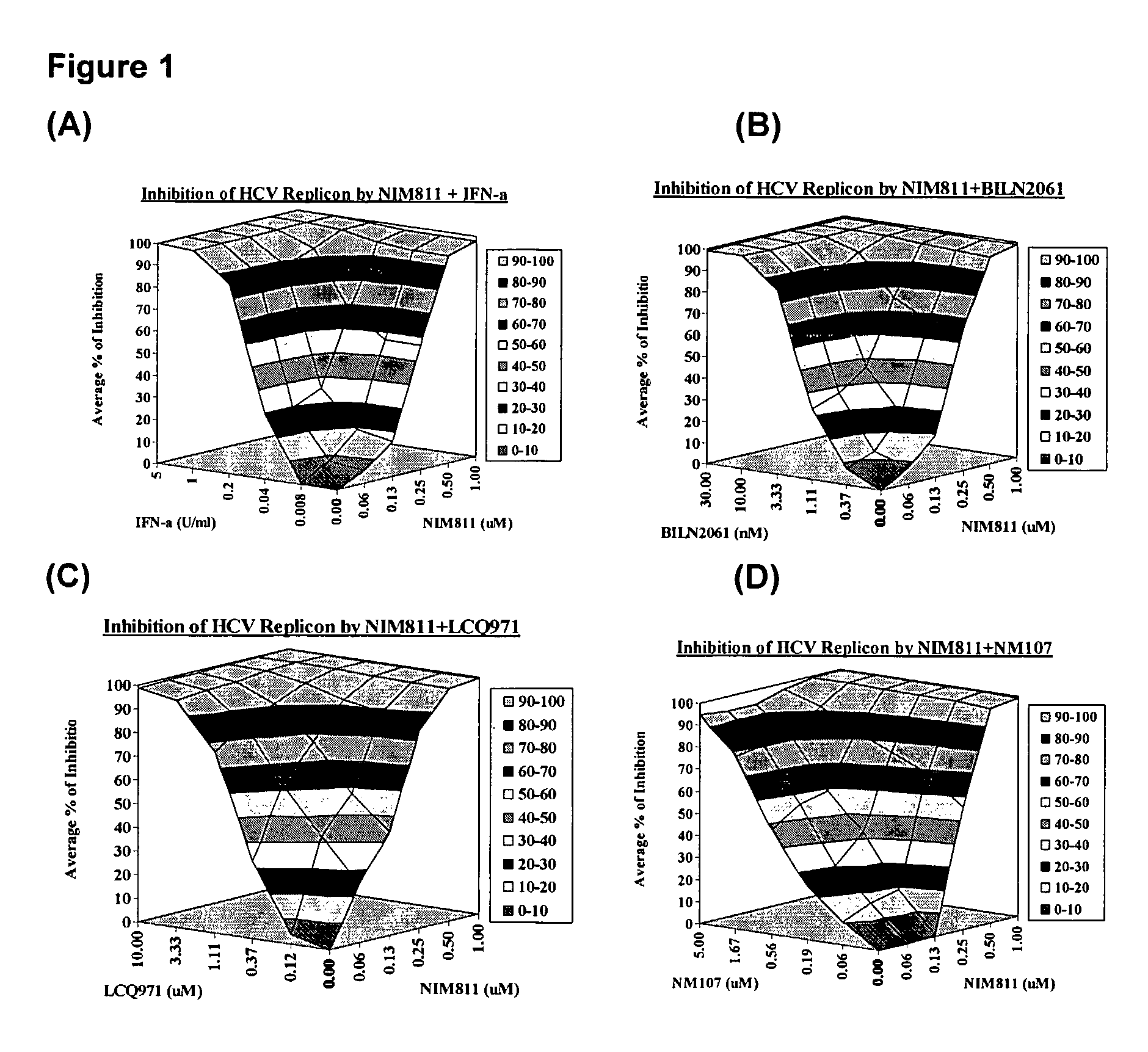
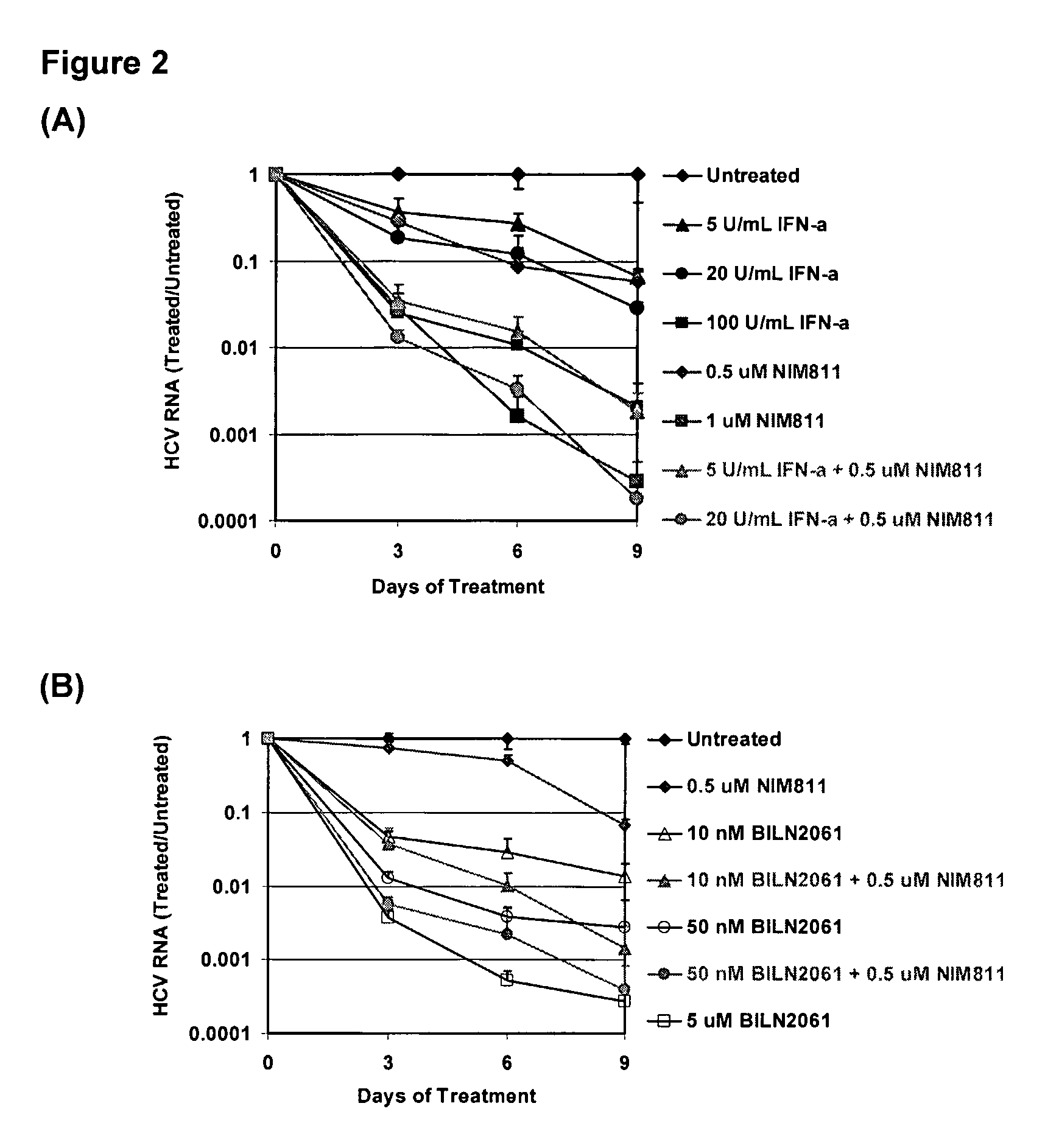
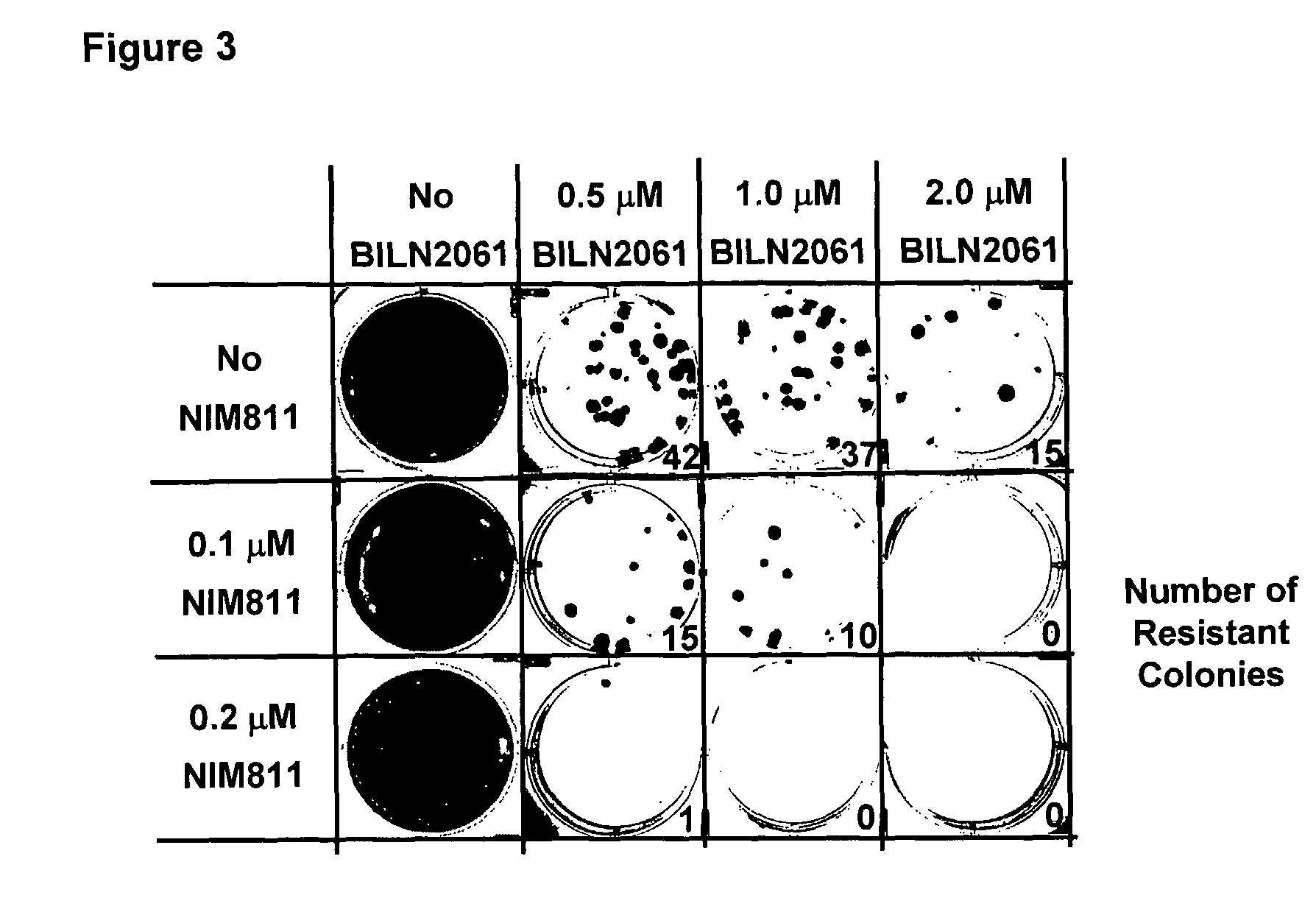
The present invention concerns a pharmaceutical combination comprising a) a first agent which is a non-immunosuppressive cyclophilin-binding cyclosporine, e.g., a compound of formula I and b) a co-agent. Co-agents include, but are not limited to, interferons, a conjugate of interferon, antiviral agents, helicase inhibitors, protease inhibitors, polymerase inhibitors and nucleoside analogs. The instant pharmaceutical combination may be used, e.g., in treating subjects having a flaviviridae infection, e.g., a Hepatitis C infection.
Owner:NOVARTIS AG
2'-C-methyl-3'-O-L-valine ester ribofuranosyl cytidine for treatment of flaviviridae infections
InactiveUS20070275883A1Improve oral bioavailabilityReduce competitionBiocidePeptide/protein ingredientsMedicineRNA-dependent RNA polymerase
The 3′-L-valine ester of β-D-2′-C-methyl-ribofuranosyl cytidine provides superior results against flaviviruses and pestiviruses, including hepatitis C virus. Based on this discovery, compounds, compositions, methods and uses are provided for the treatment of flaviviridae, including HCV, that include the administration of an effective amount of val-mCyd or its salt, ester, prodrug or derivative, optionally in a pharmaceutically acceptable carrier. In an alternative embodiment, val-mCyd is used to treat any virus that replicates through an RNA-dependent RNA polymerase.
Owner:INDENIX PHARM LLC +3
Method for the diagnosis or the screening of an arbovirus infection, reagents useful in said method and their applications
InactiveCN101784561ALow affinitySimplify Diagnostic TestingSsRNA viruses positive-senseVirus peptidesHybrid proteinImmune complex
Method for the diagnosis or the screening of an arbovirus infection and preferably a flaviviridae infection and more preferably a flavivirus infection, reagents useful in said method and their applications. Said method comprises: (i) contacting a sample from the subject or animal with a solid support sensitized with an Ig binding protein which is directed against a specific class of Ig molecules of the subject or animal species under consideration and (ii) incubating the immunocomplex formed in (i) with a detector molecule consisting of a hybrid protein comprising at least an arboviral ED3 domain and an alkaline phosphatase (PhoA), the detection of said immunocomplex being the sign of the presence of an arbovirus in said sample.
Owner:INST PASTEUR +1
Chimeric poly peptides and the therapeutic use thereof against a flaviviridae infection
The invention relates to building a chimeric polypeptide used for preventing or treating a Flaviviridae infection. The use of the inventive chimeric polypeptide for producing recombinant viral vectors such as a measles living viral vector is also disclosed.
Owner:INST PASTEUR +1
2', 3'-dideoxynucleoside analogues for the treatment or prevention of flaviviridae infections
A method for the treatment or prevention of Flaviviridae infections, in particular, hepatitis C virus infection, in a host, and in particular, a human, is provided that includes administering an effective amount of a β-L- or β-D-2′,3′-dideoxynucleoside or a pharmaceutically acceptable salt or prodrug thereof, optionally in a pharmaceutically acceptable diluent or excipient.
Owner:GILEAD SCI INC
Antiviral agents for treatment of flaviviridae infections
InactiveCN1527836ASugar derivativesGroup 5/15 element organic compoundsViral infectionAbnormal cells
The present invention discloses a composition and method for treating host including animals, especially human Flaviviridae (Hepavirus, Flavivirus, Pestivirus) virus infection, including BVDV and HCV infection, and abnormal cell proliferation, Administration of small molecules or pharmaceutically acceptable salts or prodrugs thereof is included.
Owner:PHARMASSET PHARMASSET
Modified 2' and 3'-nucleoside prodrugs for treating flaviviridae infections
Owner:INDENIX PHARM LLC +3
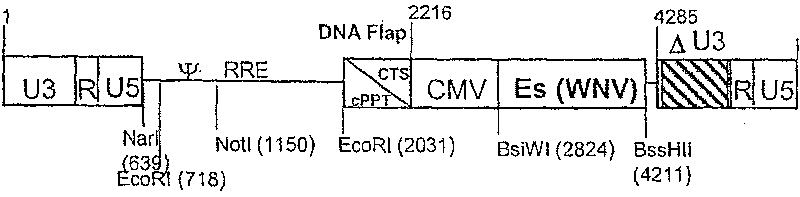
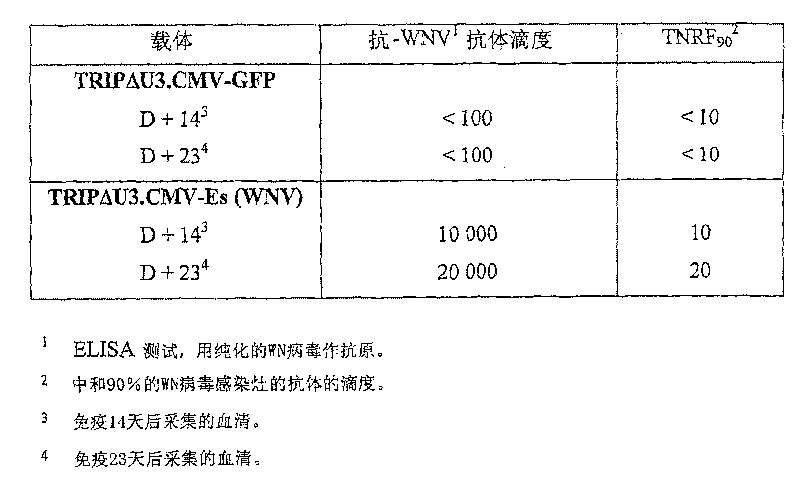
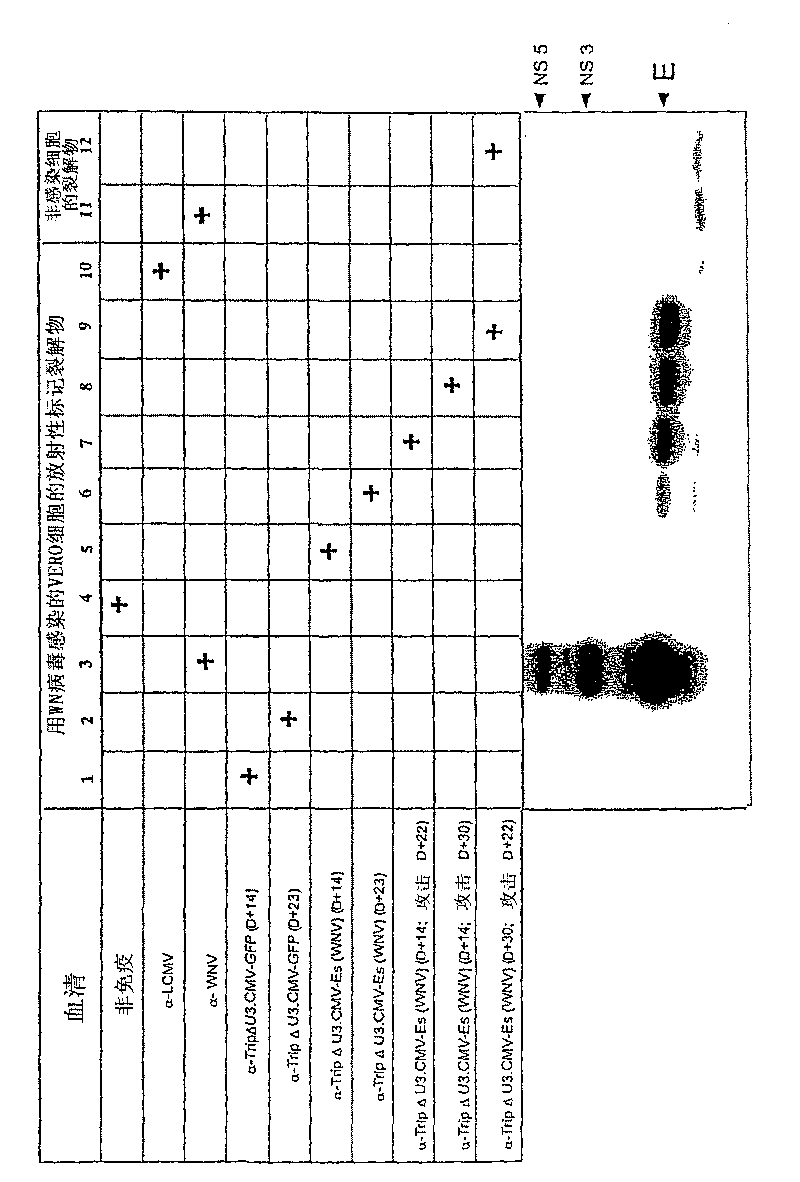
Recombinant lentiviral vector for expression of a flaviviridae protein and applications thereof as a vaccine
ActiveCN1981043BImproving immunogenicityFree from repeated administrationSsRNA viruses positive-senseGenetic material ingredientsNucleotideFamily Flaviviridae
Use of a recombinant lentiviral vector comprising a polynucleotide fragment encoding at least one protein of a virus of the family Flaviviridae or an immunogenic peptide of at least 8 amino acids of said protein, for preparing a pharmaceutical composition intended for the prevention and / or the treatment of a Flaviviridae infection in a sensitive species.
Owner:INST PASTEUR +1
Modified 2' and 3' -nucleoside produgs for treating flaviridae infections
2' and / or 3' prodrugs of l', 2', 3' or 4'-branchednucleosides, and their pharmaceutically acceptable salts and derivatives are described. These prodrugs are useful in the prevention and treatment of Flaviviridae infections, including HCV infection, and other related conditions. Compounds and compositions of the prodrugs of the present invention are described. Methods and uses are also provided that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:INDENIX PHARM LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
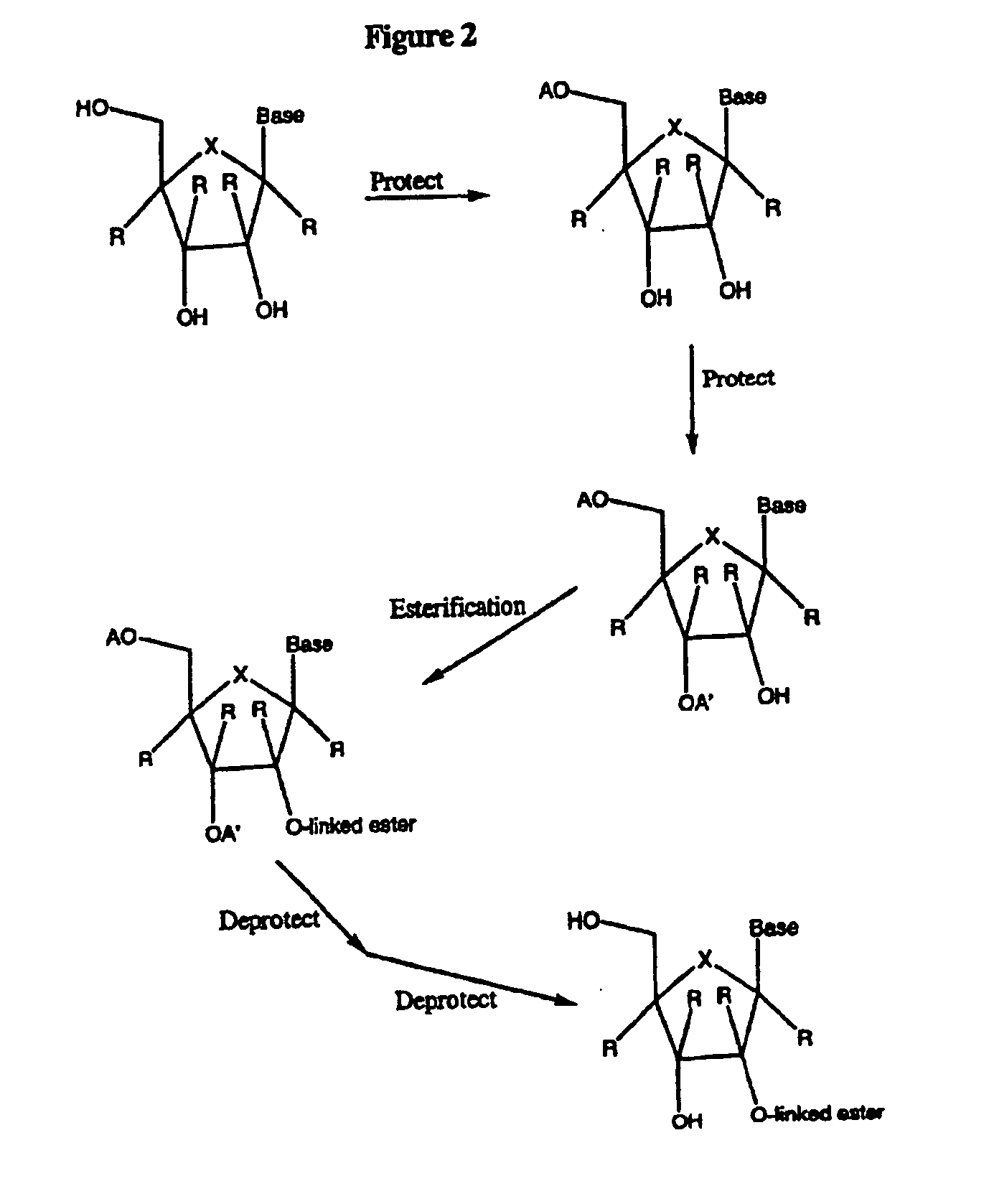
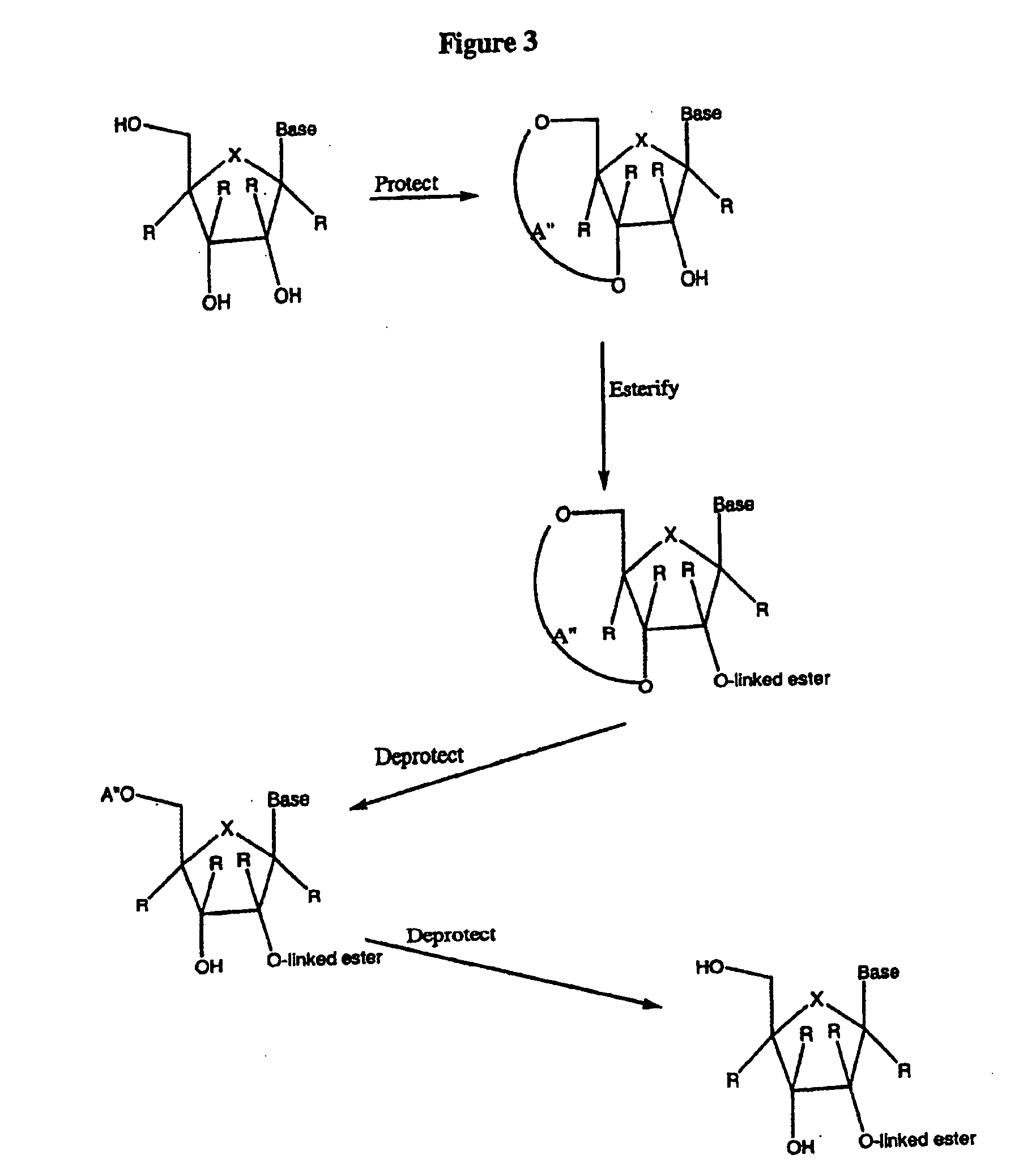
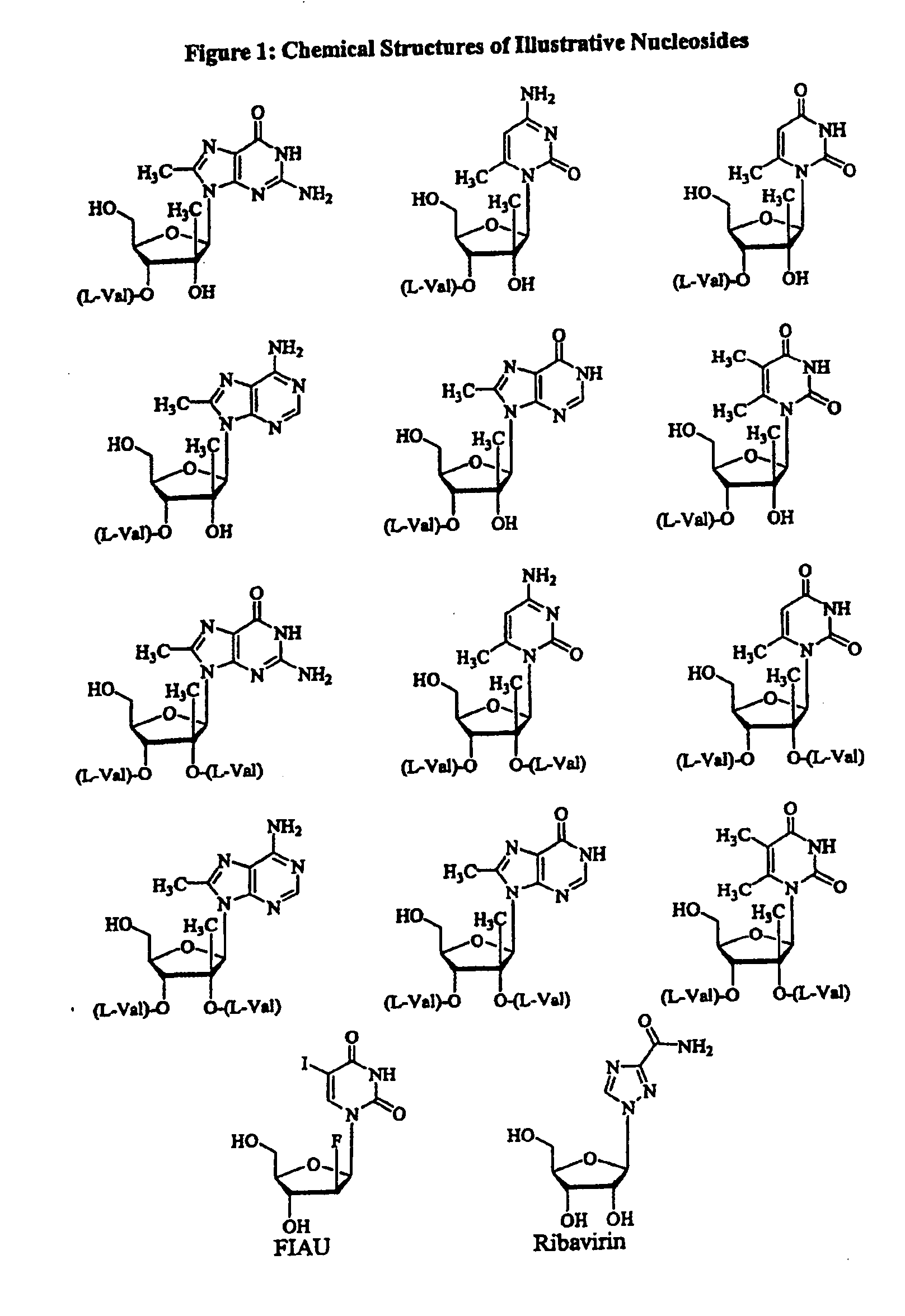
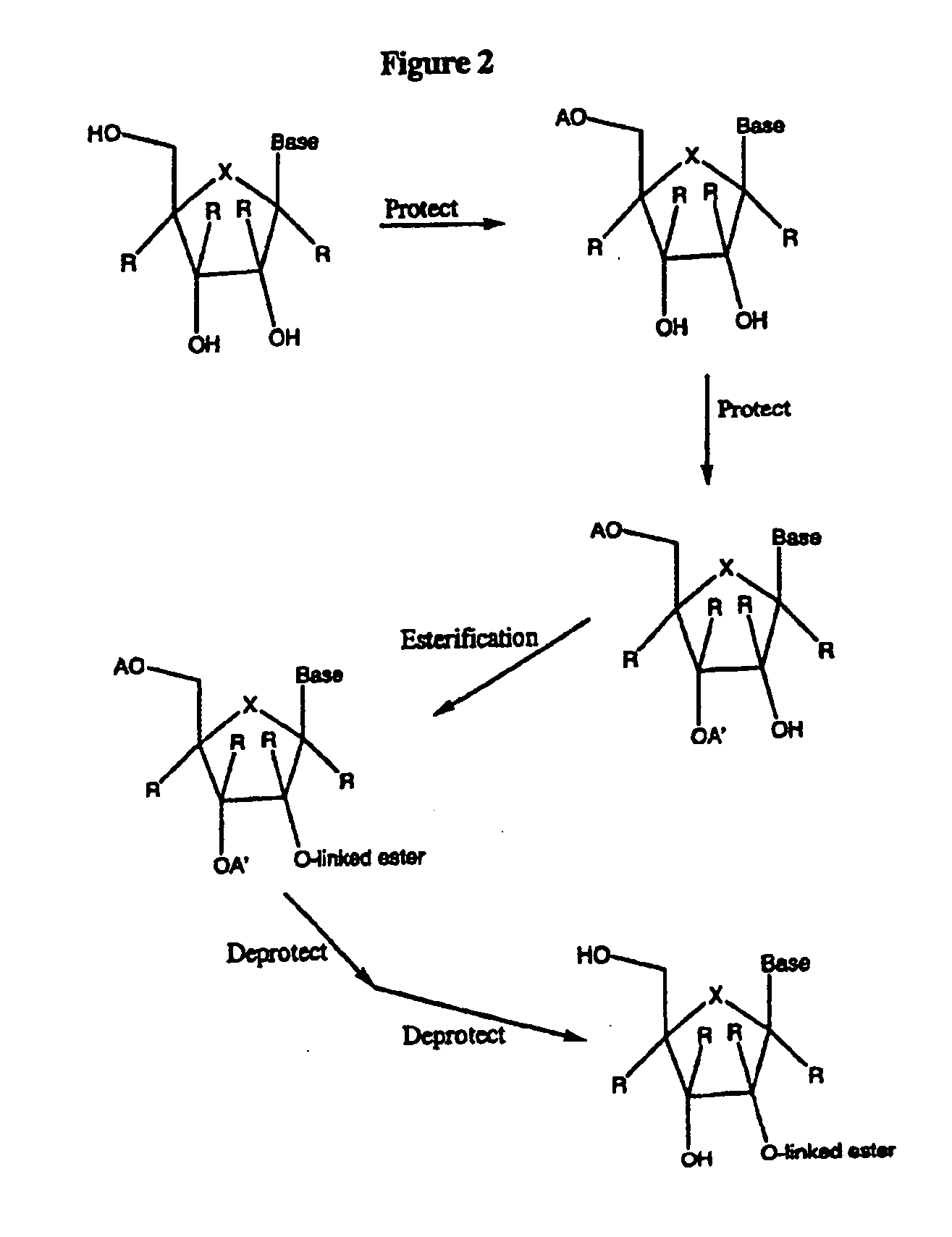
![Spiro[2.4]heptanes for treatment of flaviviridae infections Spiro[2.4]heptanes for treatment of flaviviridae infections](https://images-eureka.patsnap.com/patent_img/a47bb220-4cc9-4ee6-b785-0a4bac53bcfa/US08673926-20140318-D00001.png)
![Spiro[2.4]heptanes for treatment of flaviviridae infections Spiro[2.4]heptanes for treatment of flaviviridae infections](https://images-eureka.patsnap.com/patent_img/a47bb220-4cc9-4ee6-b785-0a4bac53bcfa/US08673926-20140318-D00002.png)
![Spiro[2.4]heptanes for treatment of flaviviridae infections Spiro[2.4]heptanes for treatment of flaviviridae infections](https://images-eureka.patsnap.com/patent_img/a47bb220-4cc9-4ee6-b785-0a4bac53bcfa/US08673926-20140318-D00003.png)