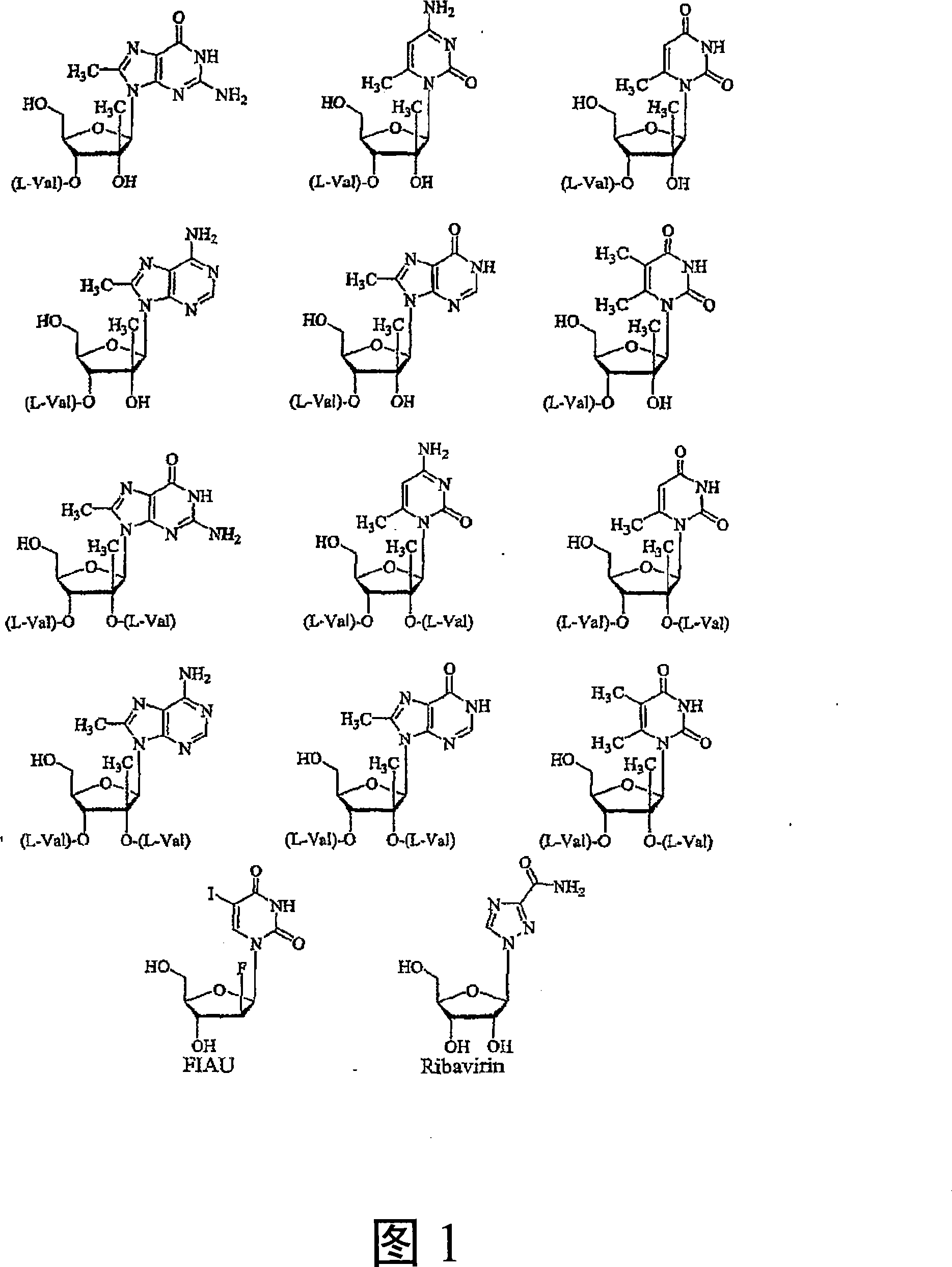
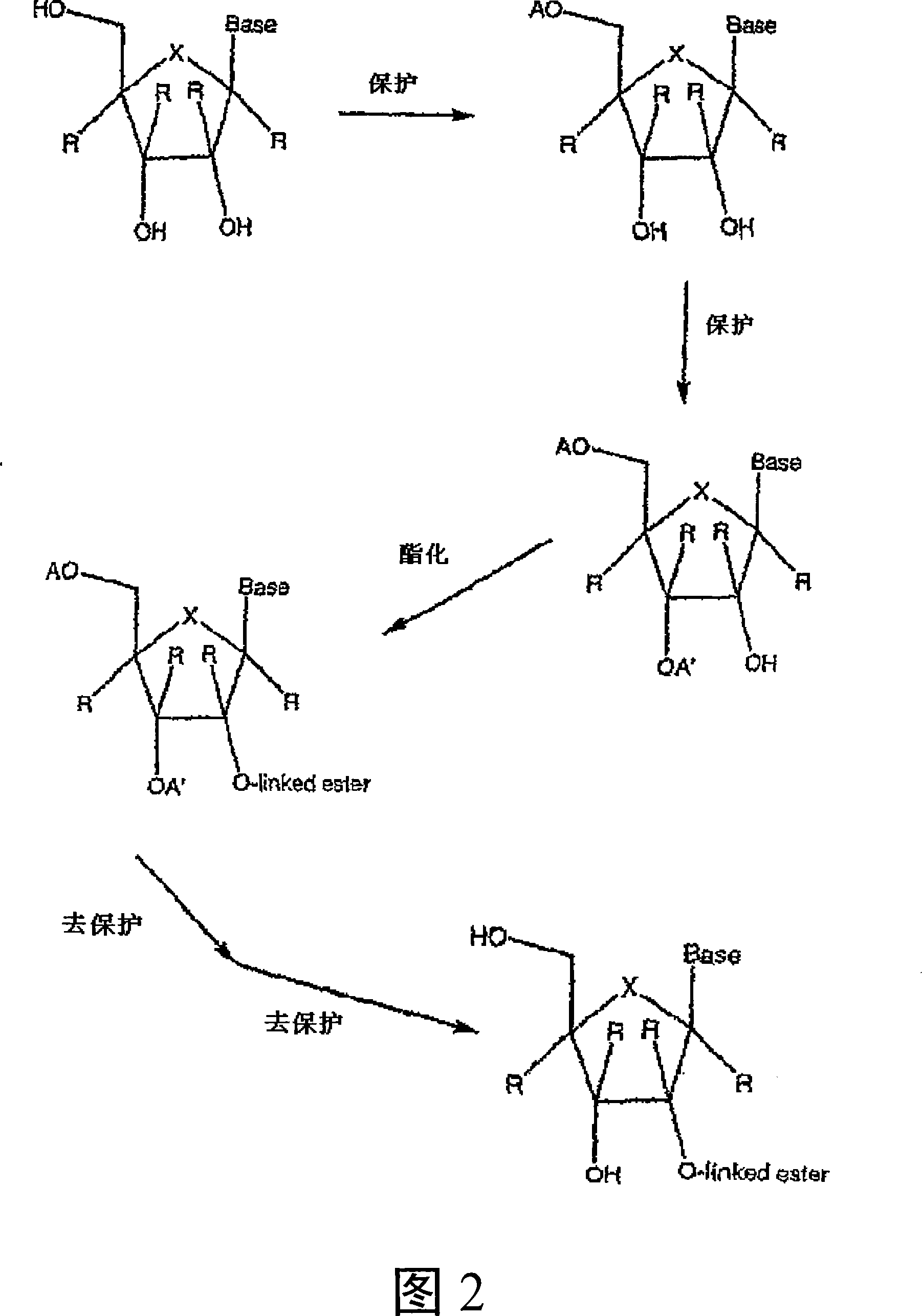
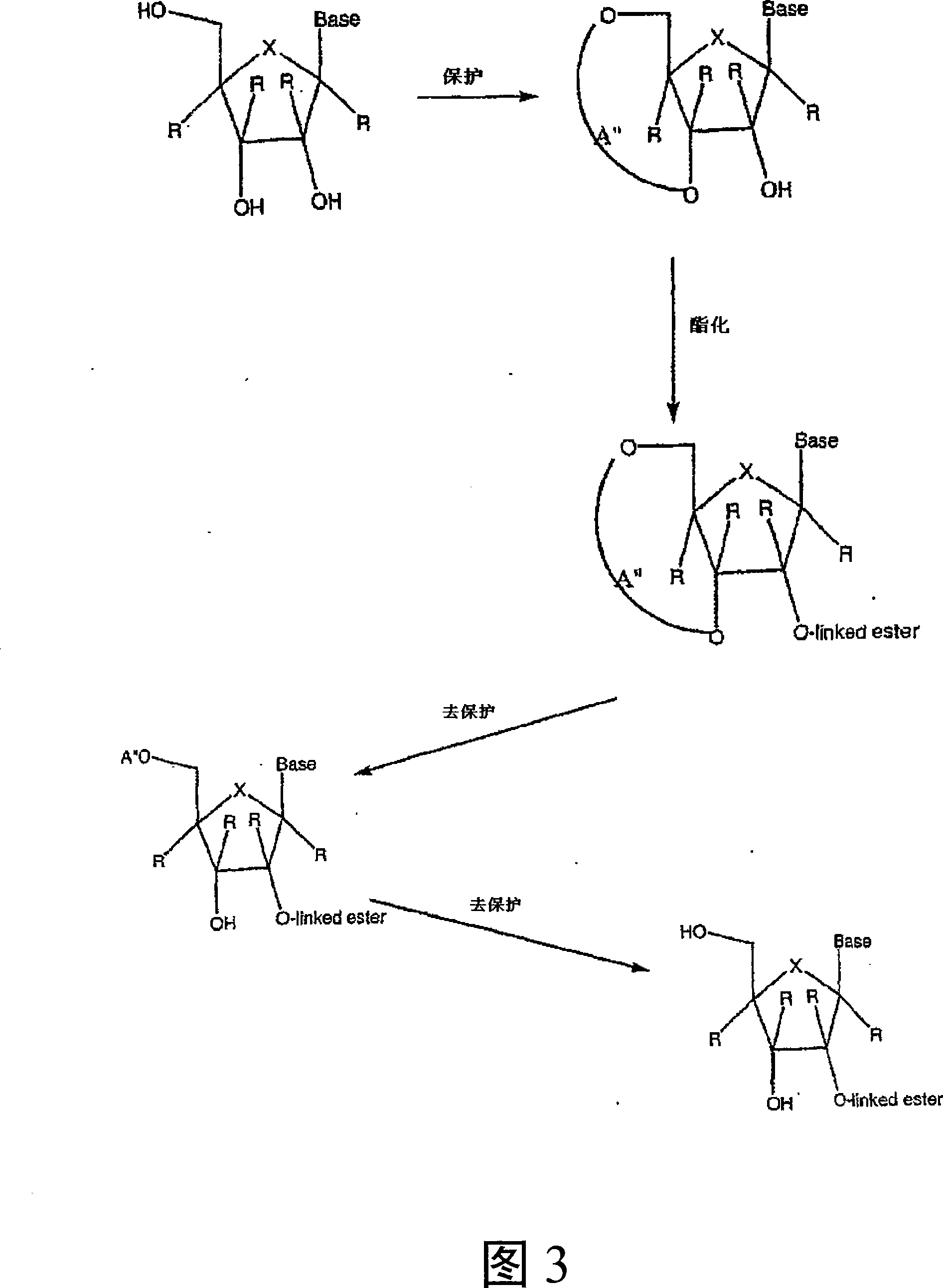
Modified 2' and 3'-nucleoside prodrugs for treating flaviviridae infections
A technology of medicinal salts and compounds, applied in the field of 2' and/or 3' prodrugs, which can solve problems such as large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0744] Non-limiting examples include:
[0745] 1) Protease inhibitors
[0746] Examples include substrate-based NS3 protease inhibitors (Attwood et al, Antiviral peptide derivatives (antiviral peptide derivatives), PCT WO 98 / 22496, 1998; Attwood et al., Antiviral Chemistry and Chemotherapy 1999, 10, 259-273; Attwood et al. ., Preparation and use of amino acid derivatives as anti-viralagents (preparation and use as an amino acid derivative of an antiviral agent), German Patent Publication No. DE 19914474; Tunget al., Inhibitiors of serine proteases, particularly hepatitis C virus NS3 proteases (inhibitors of serine proteases, especially hepatitis C virus NS3 protease), PCT WO 98 / 17679), including α-ketoamides and hydrazinoureas, and terminating electrophiles such as boronic acid or phosphonate (Llinas-Brunet et al, Hepatitis C inhibitor peptide analogues (hepatitis C virus inhibitor peptide analogs), PCT WO 99 / 07734); non-substrate-based NS3 protease inhibitors such as 2,4,6-t...
Embodiment 1
[0856] Example 1: Preparation of 1′-C-methyl riboadenine by 6-amino-9-(1-deoxy-β-D-psicofuranosyl) purine
[0857] Melting points were determined on a Mel-temp II apparatus and are uncorrected. On a Bruker 400 AMX spectrometer, a 400 MHz 1 H NMR and 100MHz 13 C NMR, NMR spectra were recorded with TMS as internal standard. Chemical shifts (δ) are expressed in parts per million (ppm) and signals are expressed as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), or bs (broad singlet). Infrared (IR) spectra were measured on a Nicolet 510P Fourier transform infrared (FT-IR) spectrometer. Mass spectra were recorded on a Micromass Autospec high resolution mass spectrometer. Thin layer chromatography (TLC) analyzes were performed on Uniplates (silica gel) from Analtech Co. Flash column chromatography was performed using silica gel-60 (220-440 mesh) or vacuum flash chromatography using silica gel G (TLC grade, >440 mesh). Ultraviolet (UV) spectra were obtained b...
Embodiment 2
[0880] Example 2: Preparation of 2'-C-methylribose-8-methyladenine
[0881] The target compound can be obtained according to the published method (R.E.Harry-O'kuru, J.M.Smith, and M.S.Wolfe, "A short, flexible route toward 2'-C-branched ribonucleosides", J.Org.Chem.1997, 62 , 1754-1759) prepared. (Process 9).
[0882] Process 9
[0883]
[0884] (a) Dess-Martin periodinane; (b) MeMgBr / TiCl 4 ; (c) BzCl, DMAP, Et 3 N; (d) bis(trimethylsilyl)acetamide, N 6 - Benzoyl adenine, TMSOTf; (e)NH 3 / MeOH
[0885]The 3'-prodrug of the 2'-branched nucleoside can be prepared according to the published method (Syntetic Communications, 1978, 8(5), 327-333; J.Am.Chem.Soc., 1999, 121(24) , 5661-5664) preparation. Alternatively, the 2'-branched nucleosides can be esterified without protection (Scheme 9b). Carbonyldiimidazole (377 mg, 2.33 mmol) was added to 15 ml of a solution of N-(tert-butoxycarbonyl)-L-valine (507 mg, 2.33 mmol) in anhydrous THF. The mixture was stirred at 20°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com