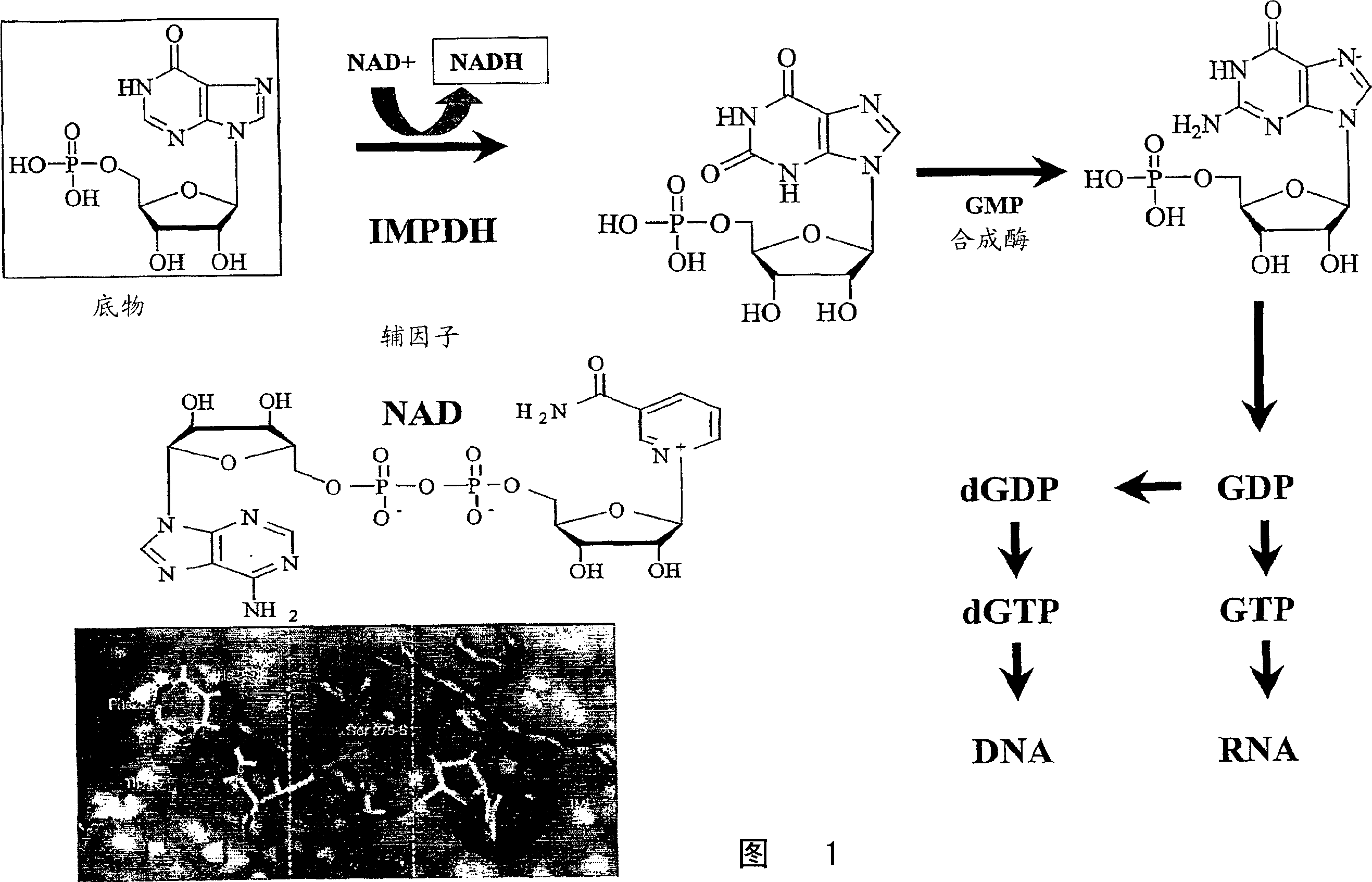
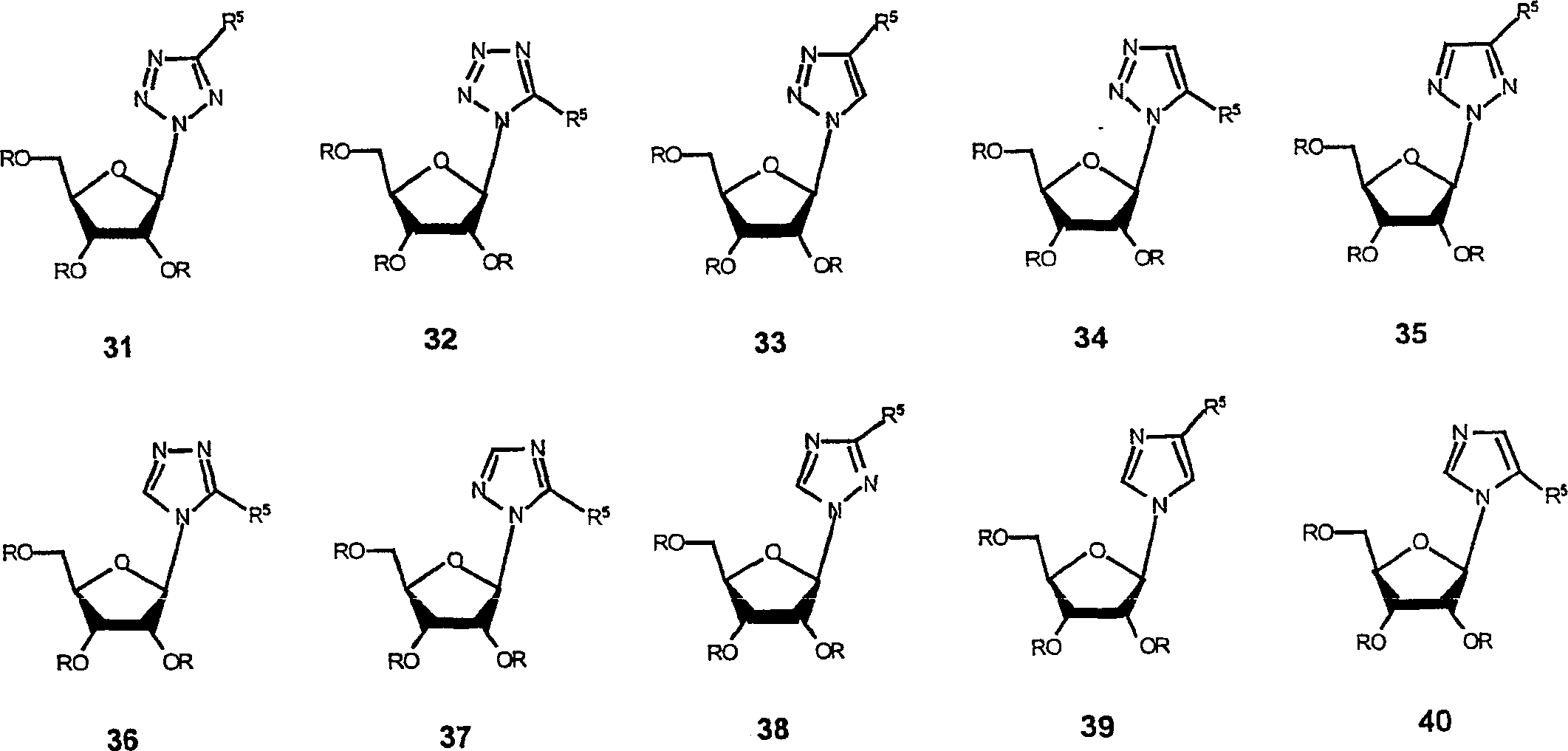
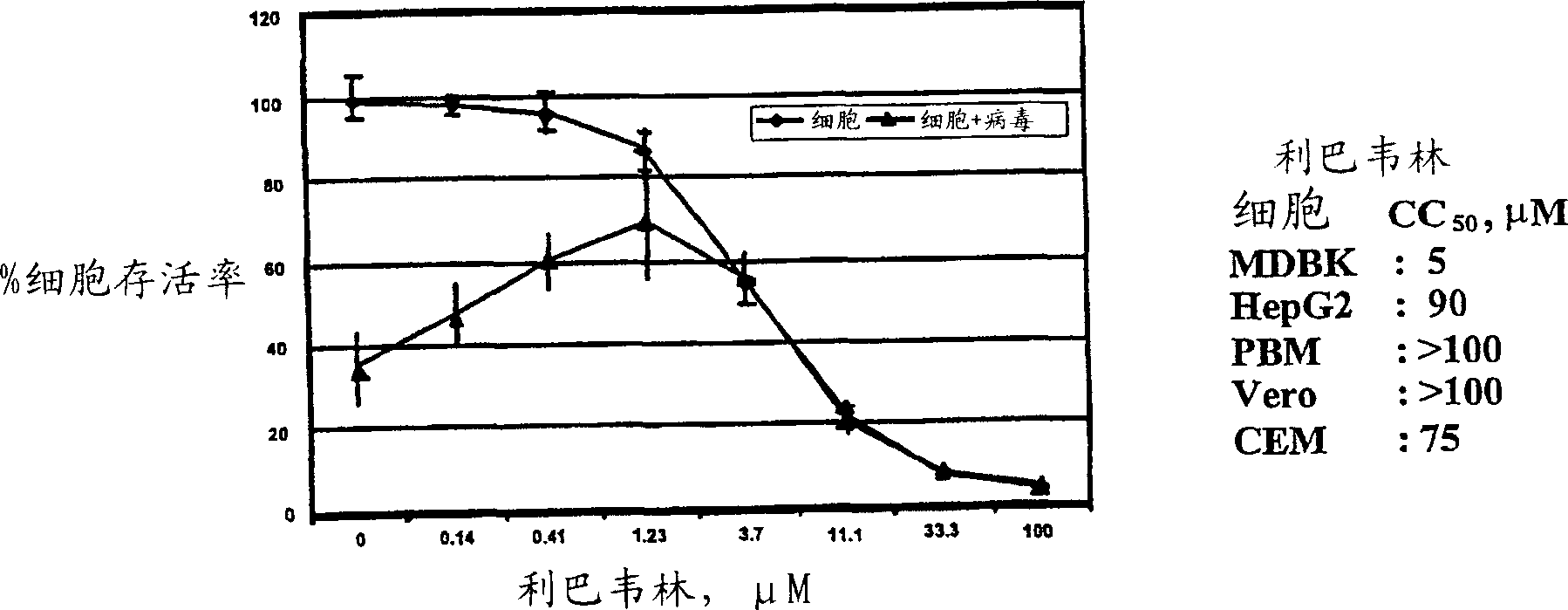
Antiviral agents for treatment of flaviviridae infections
A kind of technology of medicinal salts and compounds, applied in the field of antiviral agents for treating Flaviviridae virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0284] 5-(2,4; 3,5-di-O-benzylidene-D-hexitol (hexityl))-3-bromopyridine (13 and 14, R=H, R 5 =Br,W 6 = N, W 5 =W 7 =W 8 =CH).
[0285] To a solution of 3,5-dibromopyridine (1.45 g, 6.12 mmol) in anhydrous ether (50 mL) was slowly added (about 10 minutes) n-butyllithium (2.35 mL, 2.6M in hexane, 6.12mmol). After the addition was complete, the mixture was stirred for an additional 15 minutes. The mixture was then cooled to -78°C and a solution of 2,4;3,5-di-O-benzylidene-D-aldehyde-ribose (12,500 mg, 1.53 mmol) in tetrahydrofuran (5 mL) was added dropwise, and The reaction mixture was warmed to room temperature. Water (50 mL) was added to the reaction mixture. The organic layer was separated, washed with brine (30 mL×3), dried over sodium sulfate, and concentrated in vacuo. The residue was chromatographed on silica gel (20 g) using first dichloromethane and then 10% diethyl ether in dichloromethane as eluent to give a pale yellow product which crystallized in ethanol (...
Embodiment 2
[0330] 5-(2,4; 3,5-Di-O-benzylidene-D-hexitolyl)nicotinic acid methyl ester (13 and 14, R=H, R 5 =CO 2 CH 3 , W 6 = N, W 5 =W 7 =W 8 =CH)
[0331] Under argon atmosphere, at -78°C, to 5-(2,4; 3,5-di-O-benzylidene-D-hexitol)-3-bromopyridine (200mg, 0.43mmol) To a solution of mixed hexamethylphosphoric triamide (0.5 mL) and diethyl ether (5 mL) was added butyllithium (2 mL of 2.5M in n-hexane, 5 mmol). After the addition was complete, the mixture was stirred at -78°C for 15 minutes. A large excess of solid carbon dioxide was added and the mixture was allowed to warm to room temperature. 1N hydrochloric acid was added to acidify the mixture to pH 4, the organic layer was washed with brine (3 x 5 mL), dried over sodium sulfate. After removing sodium sulfate by filtration, the filtrate was cooled to 0°C and washed with a large excess of diazomethane in ether. Acetic acid was then added to destroy excess diazomethane. The mixture was concentrated in vacuo, and the residue...
Embodiment 3
[0344] Methyl 5-(2,4; 3,5-di-O-benzylidene-D-hexitolyl)nicotinamide (13 and 14, R=H, R 5 =CONH 2 , W 6 = N, W 5 =W 7 =W 8 =CH).
[0345] The allose / altrose form of 5-(2,4;3,5-di-O-benzylidene-D-hexitolyl)nicotinic acid methyl ester (220mg, 0.48mmol) was differentially The isomeric mixture was treated with saturated ammonia in methanol containing a catalytic amount of sodium hydride (ca. 2 mg), and the mixture was stirred at room temperature overnight. The solvent was removed in vacuo and the residue was chromatographed on a silica gel column with chloroform-methanol (95:5) as eluent. Methyl 5-(2,4;3,5-di-O-benzylidene-D-hexitolyl)nicotinamide was obtained as a mixture of allose-type / altrrose-type epimers ( 13 and 14, R = H, R 5 =CONH 2 , W 6 = N, W 5 =W 7 =W 8 =CH), 185mg (87%). 1 H NMR (Me 2 SO-d 6 ): δ3.5-4.3(5H, m, H-2′, 3′, 4′, 5′, 5″), 5.00(1H, m, H-1′), 5.64, 5.76, 5.77, 5.89( 2H, 4s, benzylidene-CH2 ), 8.29, 8.68, 8.95 (3H, 3m, pyridine).
[0346] Foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com