Compositions and Methods for Treating Neurogenic Disorders of the Pelvic Floor
a neurogenic disorder and pelvic floor technology, applied in the field of pelvic floor neurogenic disorders, can solve the problems of non-selective electrodes, incomplete bladder emptying, and “reflux” of urine into the kidneys, and achieve the effect of depolarization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Use of Optogenetic-Based Nerve Stimulation in an Animal Model of Detrusor External Sphincter Dysnergia (DSD) and Detrusor Hyperreflexia (DH)
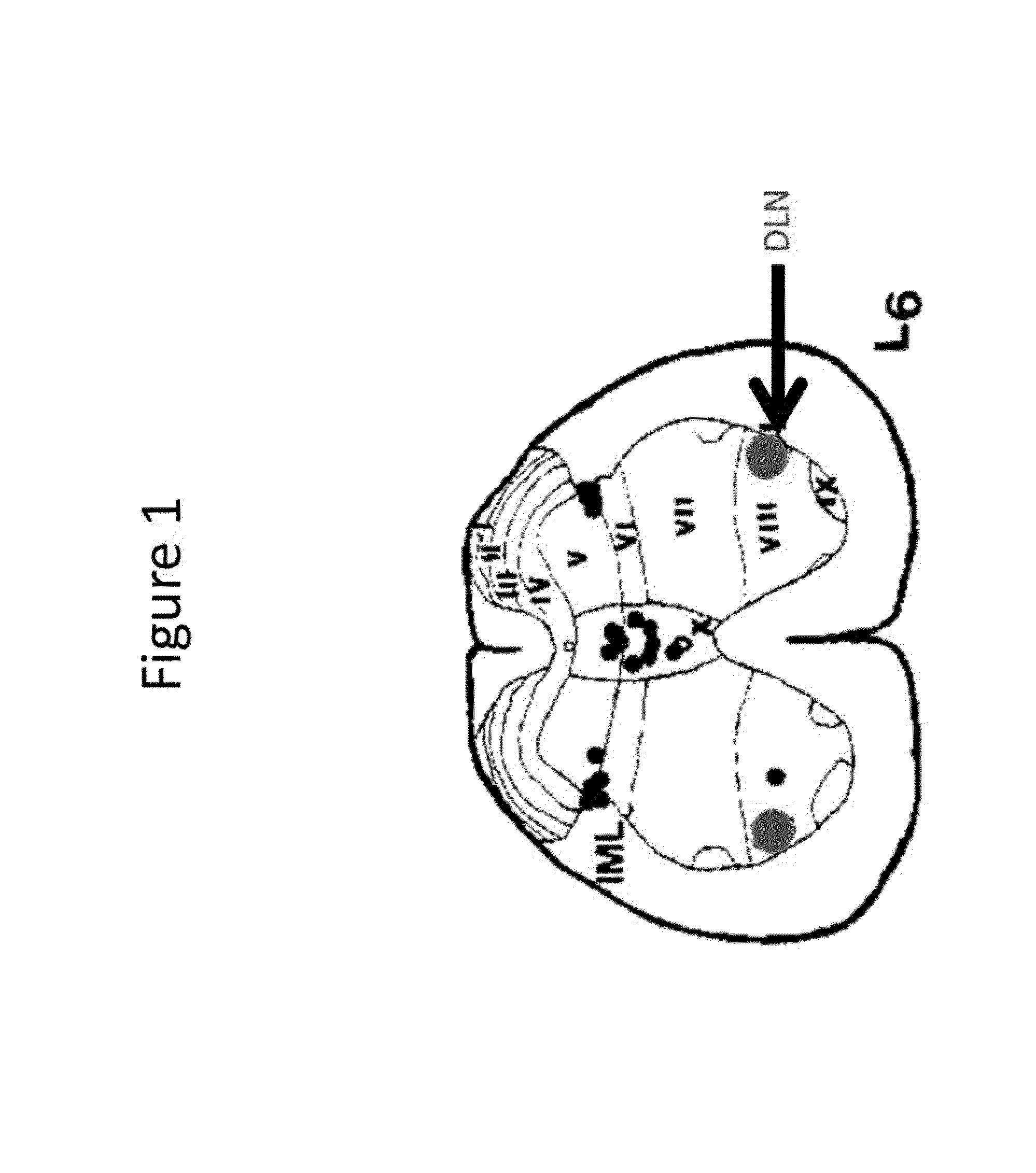
[0187]This Example validates an animal model of DSD and DH for treatment with the optogenetic methods described herein. Cat models with spinal cord injuries have been used to recreate the human conditions of DSD and DH, having been validated using PET (Tai et al., 2004, Experimental Neurol., 190:171). There are also animal models of hyperreflexia in spinal cord injured (SCI) rats (Shaker et al., 2003, Neurourol Urodyn., 22(7):693-8) as well as in the EAE mouse, which is also a model for multiple sclerosis (Vignes et al., 2007, J. Physio. 578(Pt 2):439-50). In this Example, the membrane-targeted photoactivateable anion pump halorhodopsin from Natronomonas pharaonis (NpHR) is used to hyperpolarize the nerves responsible for the innervation of the detrusor muscle of the bladder and the external urinary sphincter.
Materials and Methods
[0188]Cats with...
example 2
Use of Light-Responsive Cation Channels to Provoke Depolarization-Induced Synaptic Depletion in An Animal Model of Detrusor External Sphincter Dysnergia (DSD) and Detrusor Hyperreflexia (DH)
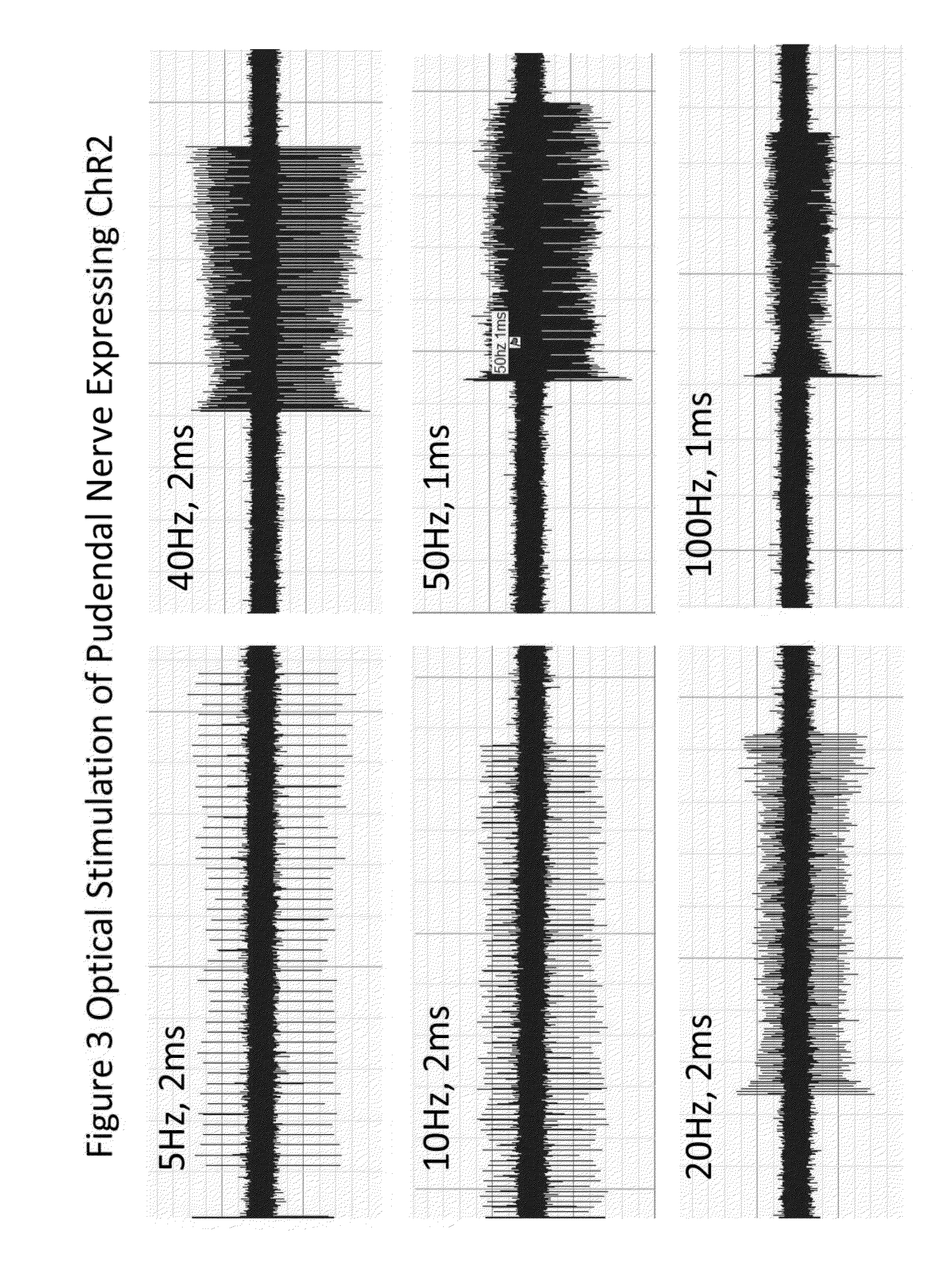
[0203]This Example validates an animal model of DSD and DH for treatment with the optogenetic methods described above whereby urinary function is restored via selective depolarization-induced synaptic depletion of the detrusor innervations arising from the sacral spinal nerves and the external urinary sphincter innervations of the pudendal nerve. The feline or rodent animal models are identical to those used in Example 1.
[0204]Yellow fluorescent protein (YFP)-labeled SSFO (pAAV-Thy1-hChR2 (E123T / T159C)-EYFP; see www(dot)optogenetics(dot)org) in an AAV1 viral vector and under control of the feline Thy1 promoter is injected directly into the somatic motor neuron cell body of the sacral spinal nerves (responsible for detrusor innervations) and into Onuf's nucleus (responsible for external urinary sp...
example 3
AAV Vector Constructs
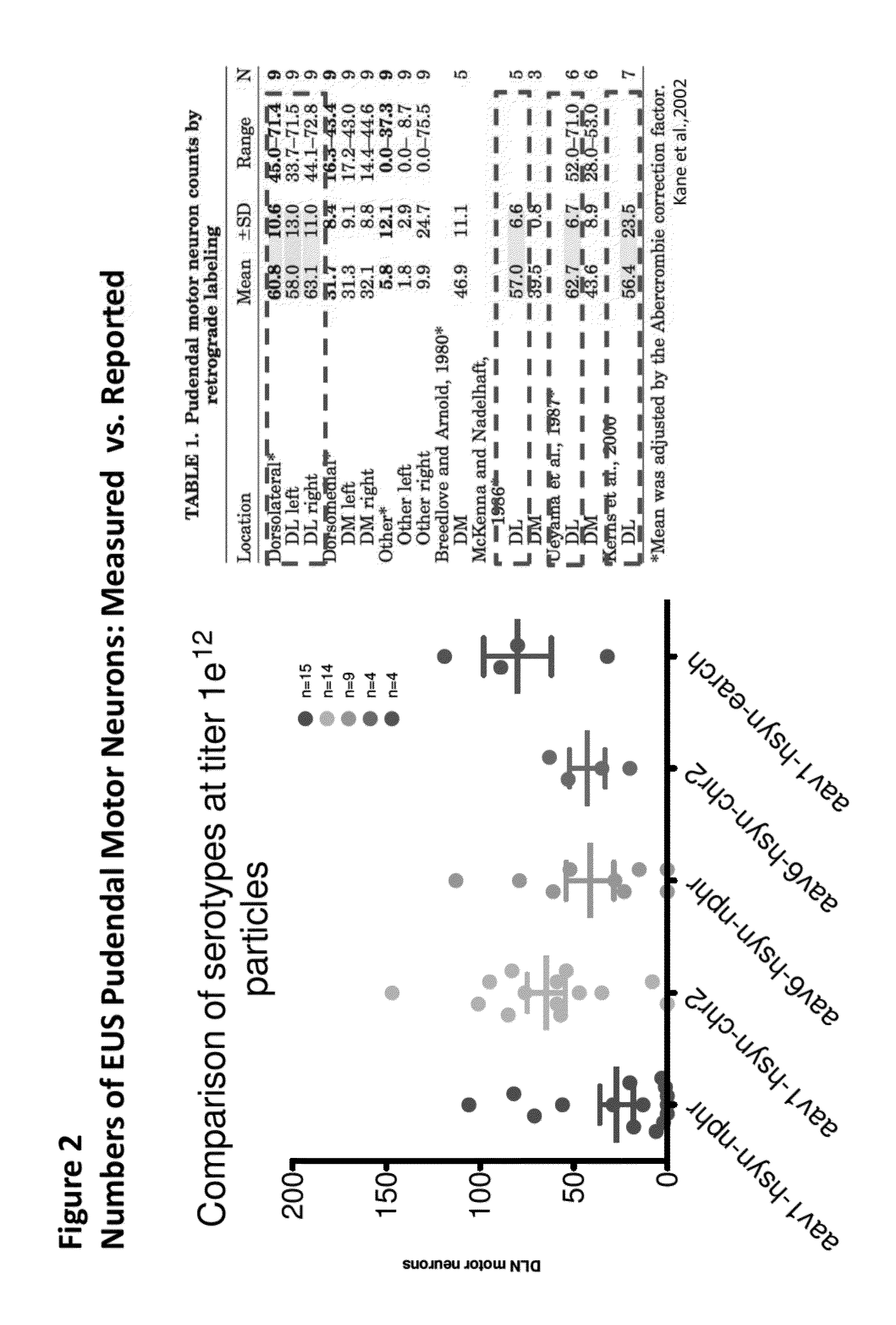
[0207]The following Adenoassociated virus (AAV) constructs were generated: 1) AAV1:hsyn-ChR2-EYFP (AAV1 comprising a nucleotide sequence encoding a ChR2-eYFP fusion protein operably linked to a human synapsin 1 promoter); 2) AAV6-hsyn-ChR2-EYFP (AAV6 comprising a nucleotide sequence encoding a ChR2-eYFP fusion protein operably linked to a human synapsin 1 promoter); 3) AAV1-hsyn-NpHR-EYFP (AAV 1 comprising a nucleotide sequence encoding an NpHR 3.0-EYFP fusion protein, operably linked to a human synapsin 1 promoter); 4) AAV6-hsyn-NpHR-EYFP (AAV6 comprising a nucleotide sequence encoding an NpHR 3.0-EYFP fusion protein, operably linked to a human synapsin 1 promoter); 5) AAV1-hsyn-eARCH-EYFP (AAV1 comprising a nucleotide sequence encoding an eARCH 3.0-EYFP fusion protein, operably linked to a human synapsin 1 promoter).
[0208]Single-stranded DNA AAV viruses were produced in a baculovirus system (Virovek, Hayward, Calif.; as described in WO 2008 / 024998
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com