Sustained release intraocular implants and methods for preventing retinal dysfunction
a technology of intraocular implants and retinal dysfunction, which is applied in the field of sustained release intraocular implants and methods for preventing retinal dysfunction, can solve the problems that the technique of administering brimonidine to the posterior chamber of the eye is not sufficient to address this issue, and achieves the effect of few or no negative side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
In Vivo Testing of Intraocular Implants Containing Brimonidine and a Biodegradable Polymer Matrix
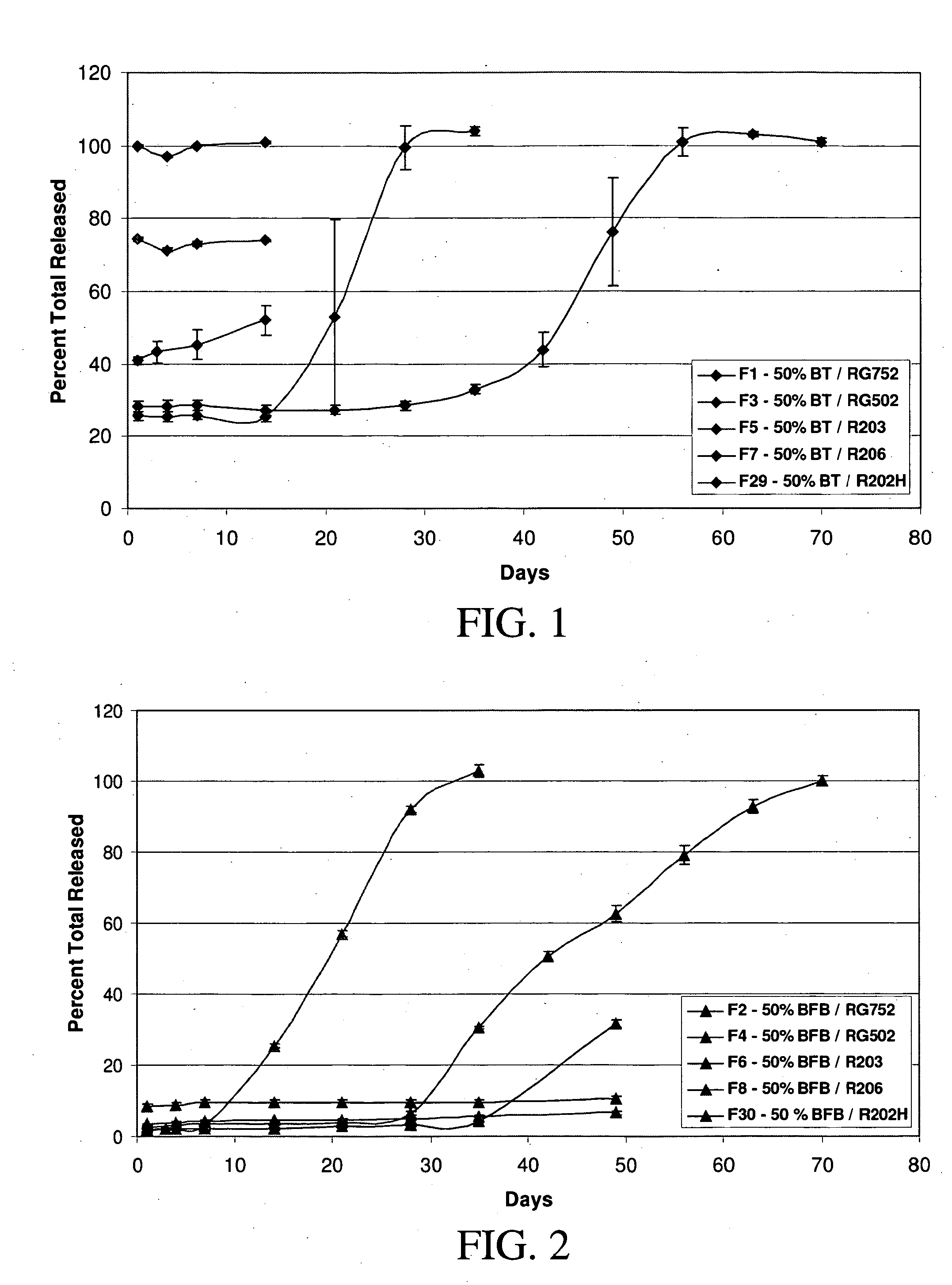
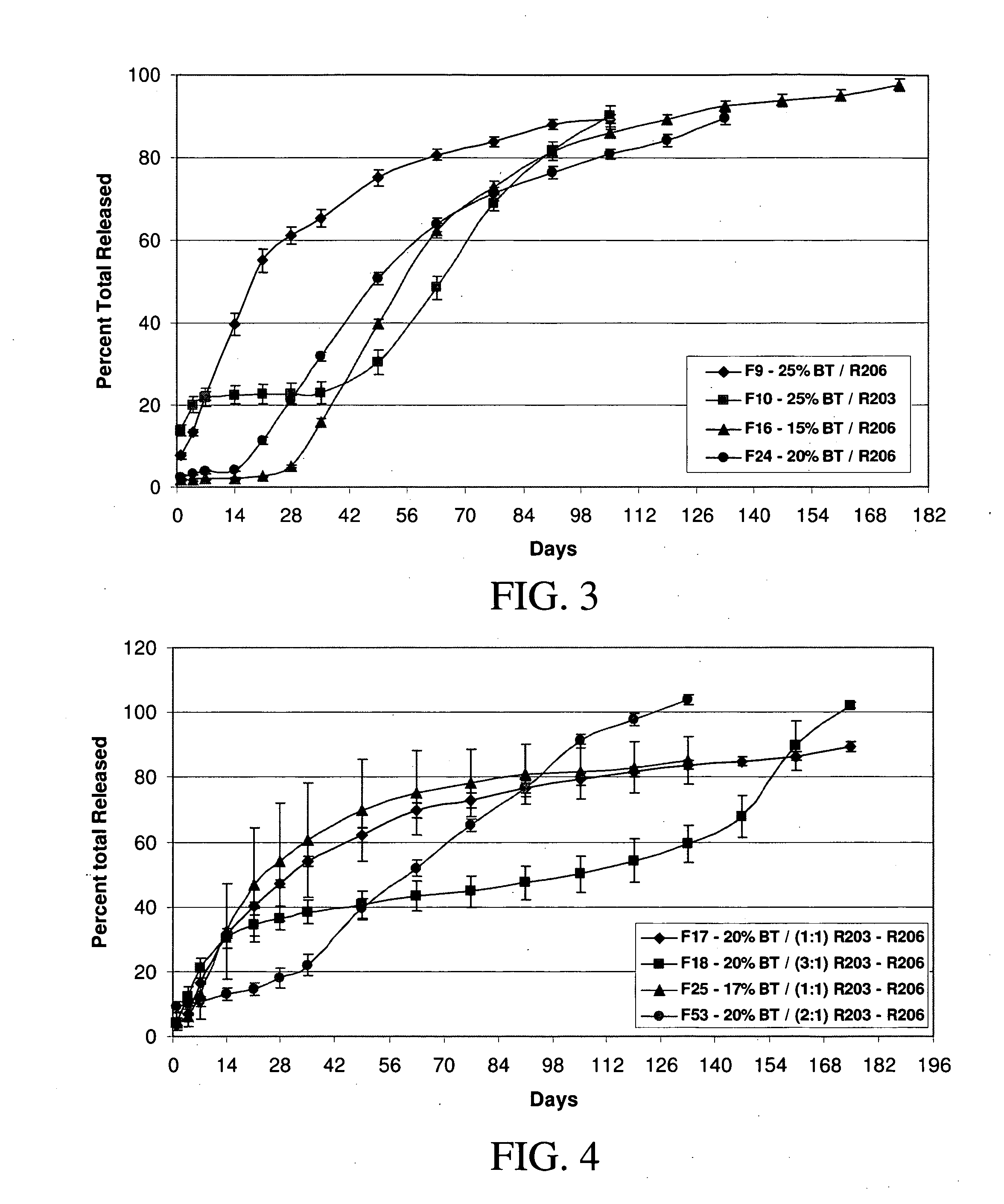
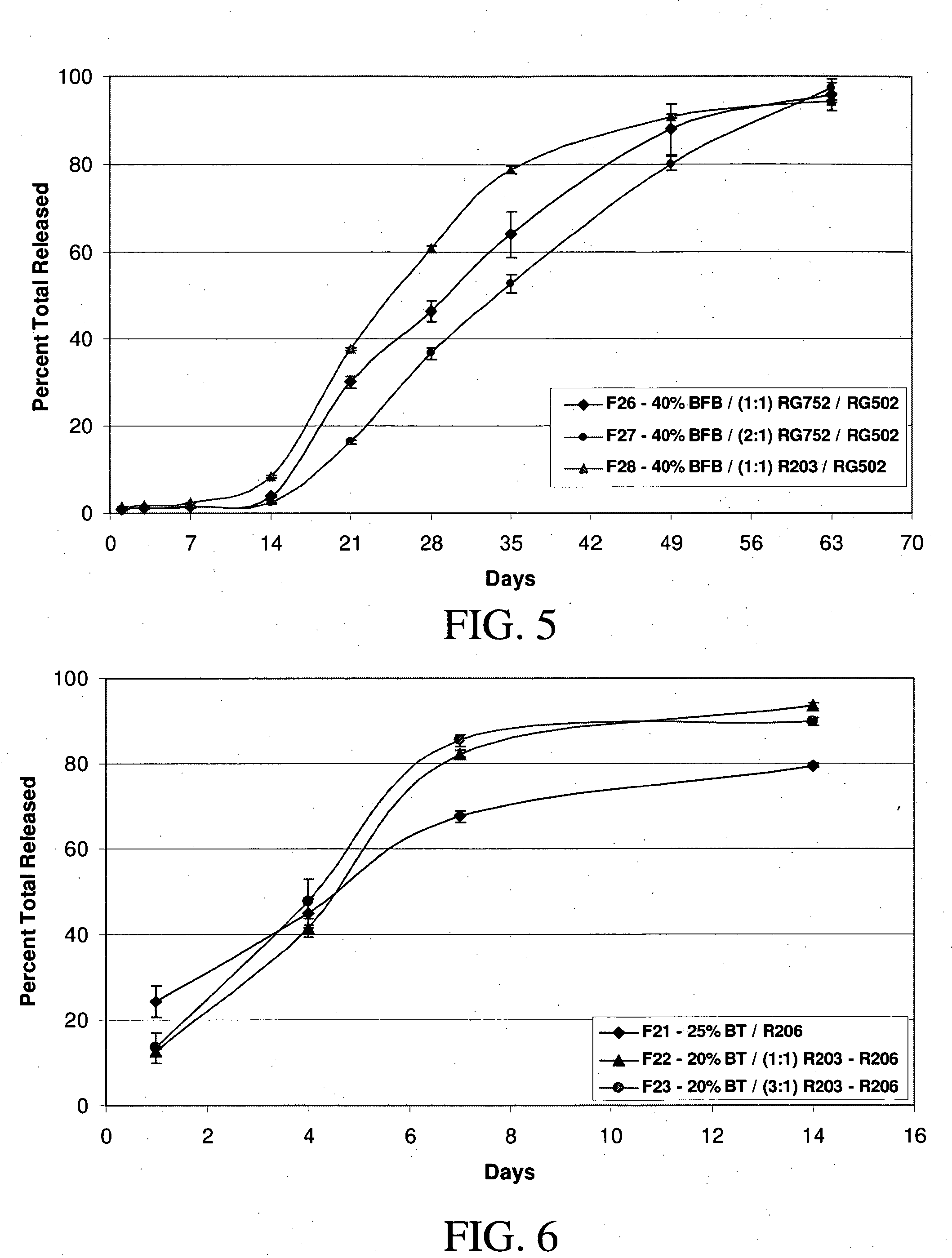
[0157] Implants containing brimonidine tartrate, such as formulation #17 described in Example 1, and placebo implants without a therapeutic agent were placed in the vitreous of normal eyes of Dutch Belt pigmented rabbits (2.0-2.5 kg). Two weeks after implantation, electroretinograms (ERGs) were measured in each of the eyes of the rabbits as follows. As understood by persons of ordinary skill in the art understand, the ERG reflects the summation of electrical responses generated by neurons and non-neuronal cells in the retina and pigment epithelium in response to light. The major ERG components are the fast negative A-wave, the fast positive B-wave and the slow, positive C-wave. The leading edge of the A-wave provides a direct measure of photoreceptor activity, while the B-wave provides a reflection of the action of glial and other cells.
[0158] The rabbits were dark adapted for more tha...
example 3
Treatment of Ocular Conditions with Various Active Agents
[0167] An implant can be formulated with various active agents, including the agents described herein, following the procedures in the Examples above. These implants can provide an extended therapeutic treatment of an ocular condition, that is a therapeutic effect during a period of time during release of the active agent or after release of all of the active agent from the implant and during which there is no longer a therapeutic amount of the active agent present at the ocular site at which the implant was placed. Thus, an implant can be prepared containing an alpha-2 adrenergic receptor agonist, such as clonidine, apraclonidine, or brimonidine (available from Allergan, Irvine, Calif. as brimonidine tartrate ophthalmic solution, under the tradename Alphagan-P®). Thus, for example, a brimonidine extended therapeutic treatment implant can be implanted into an ocular site (i.e. into the vitreous) of a patient with an ocular co...
example 4
In Vivo Testing of Intraocular Microspheres Containing Brimonidine and a Biodegradable Polymer Matrix
[0168] I. Introduction
[0169] An experiment was carried out with microspheres containing an alpha 2 agonist as the active agent. The alpha-2 agonist used was brimonidine free base. The brimonidine microspheres were injected (or synonymously implanted) into various vitreal locations of one normal eye of four separate rabbits. Thus, four mammalian eyes were injected with the brimonidine microspheres. The vitreous of each other eye of the same four rabbits was injected with Kenalog® 40. Kenalog® 40 is a triamcinolone suspension. Each milliliter of Kenalog® 40 includes 40 milligrams of triamcinolone acetonide, sodium chloride as a tonicity agent, 10 mg of benzyl alcohol as a preservative, and 7.5 mg of carboxymethylcellulose and 0.4 mg of polysorbate 80 as resuspension aids.
[0170] This experiment determined that intravitreal alpha-2 agonist microspheres, such as brimonidine microsphere...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com