Patents
Literature
81 results about "Cleanroom" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A cleanroom or clean room is a facility ordinarily utilized as a part of specialized industrial production or scientific research, including the manufacture of pharmaceutical items and microprocessors. Cleanrooms are designed to maintain extremely low levels of particulates, such as dust, airborne organisms, or vaporized particles. Cleanrooms typically have a cleanliness level quantified by the number of particles per cubic meter at a predetermined molecule measure. The ambient outdoor air in a typical urban area contains 35,000,000 particles for each cubic meter in the size range 0.5 μm and bigger in measurement, equivalent to an ISO 9 cleanroom, while by comparison an ISO 1 cleanroom permits no particles in that size range and just 12 particles for each cubic meter of 0.3 μm and smaller.
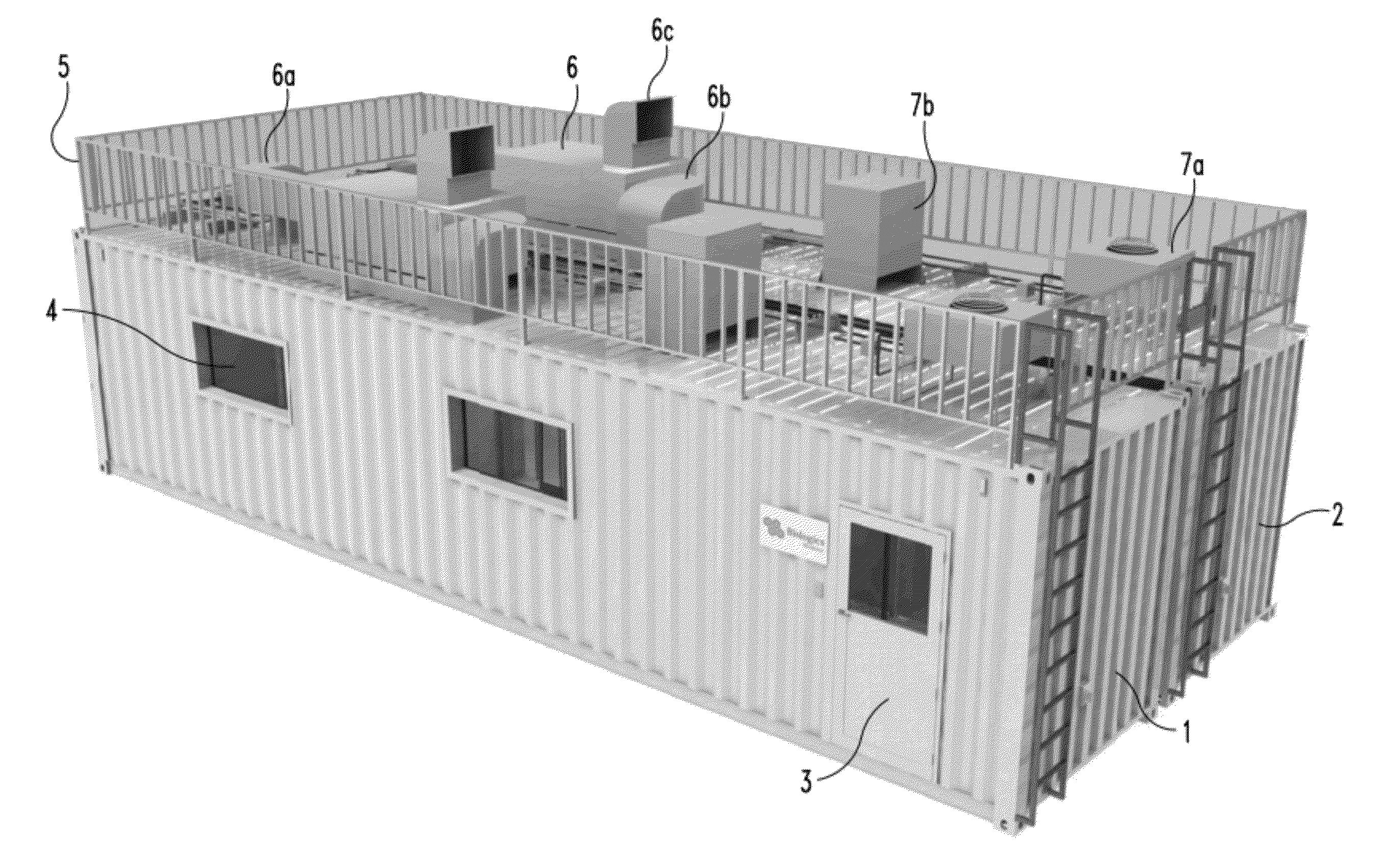
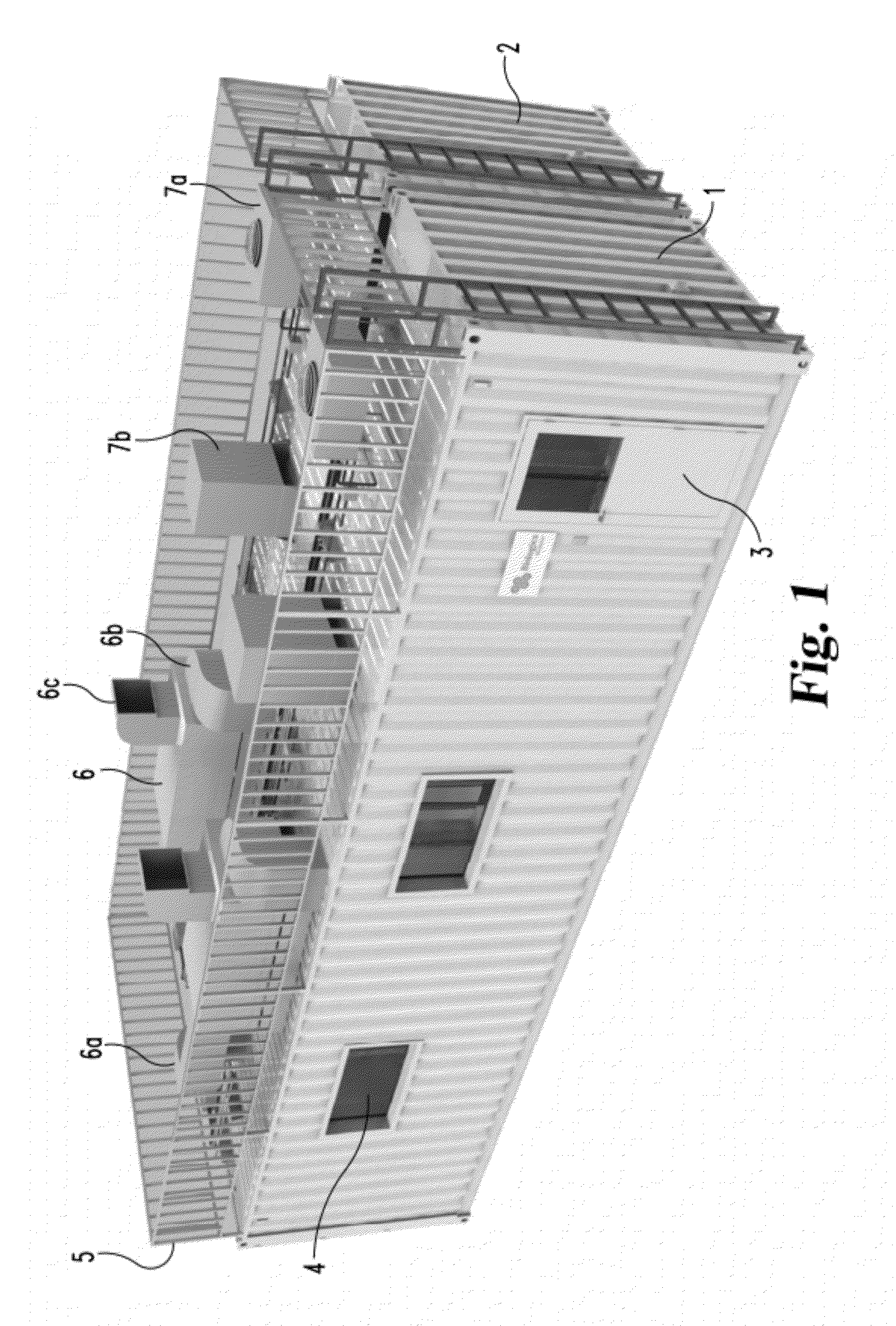
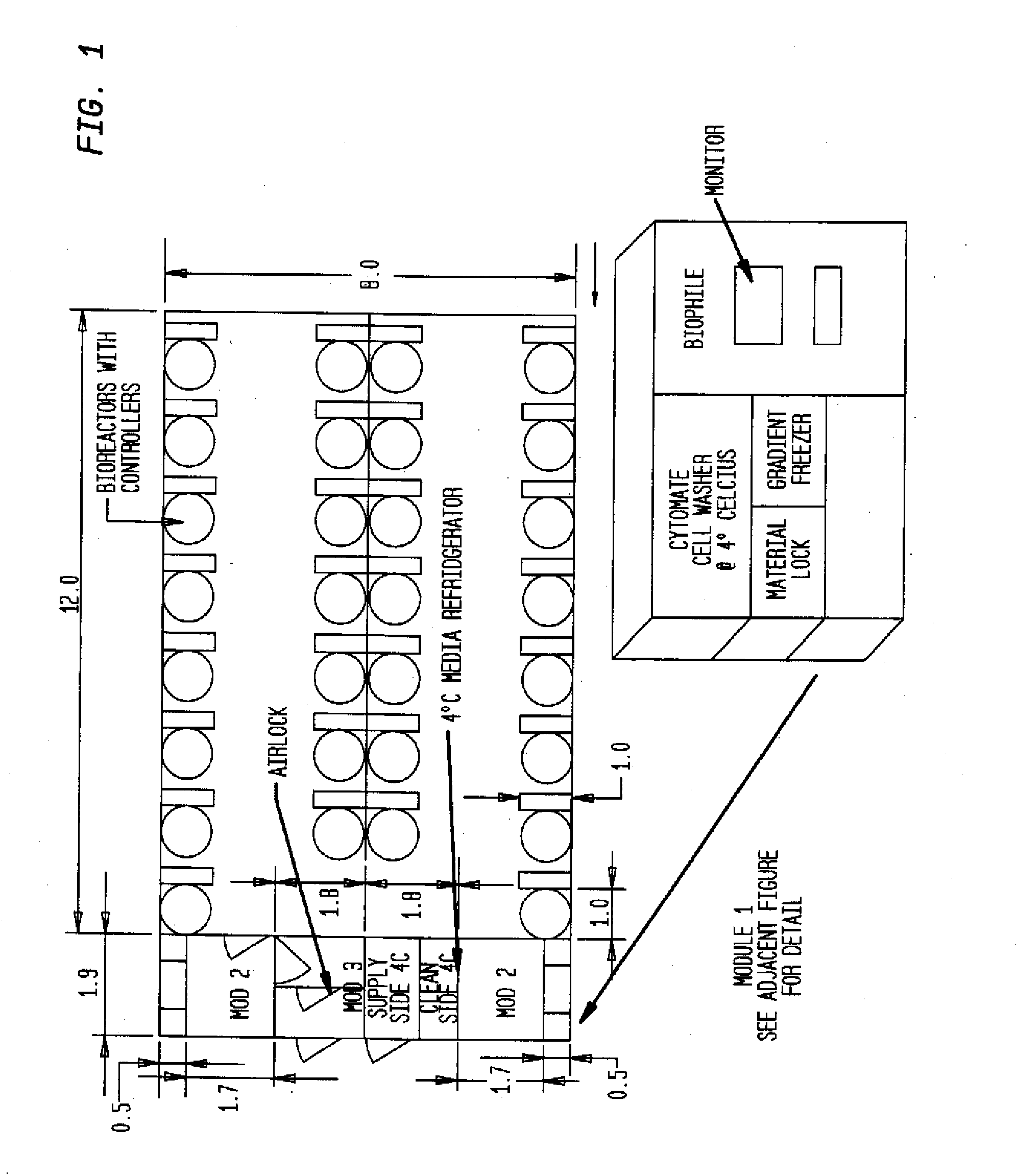
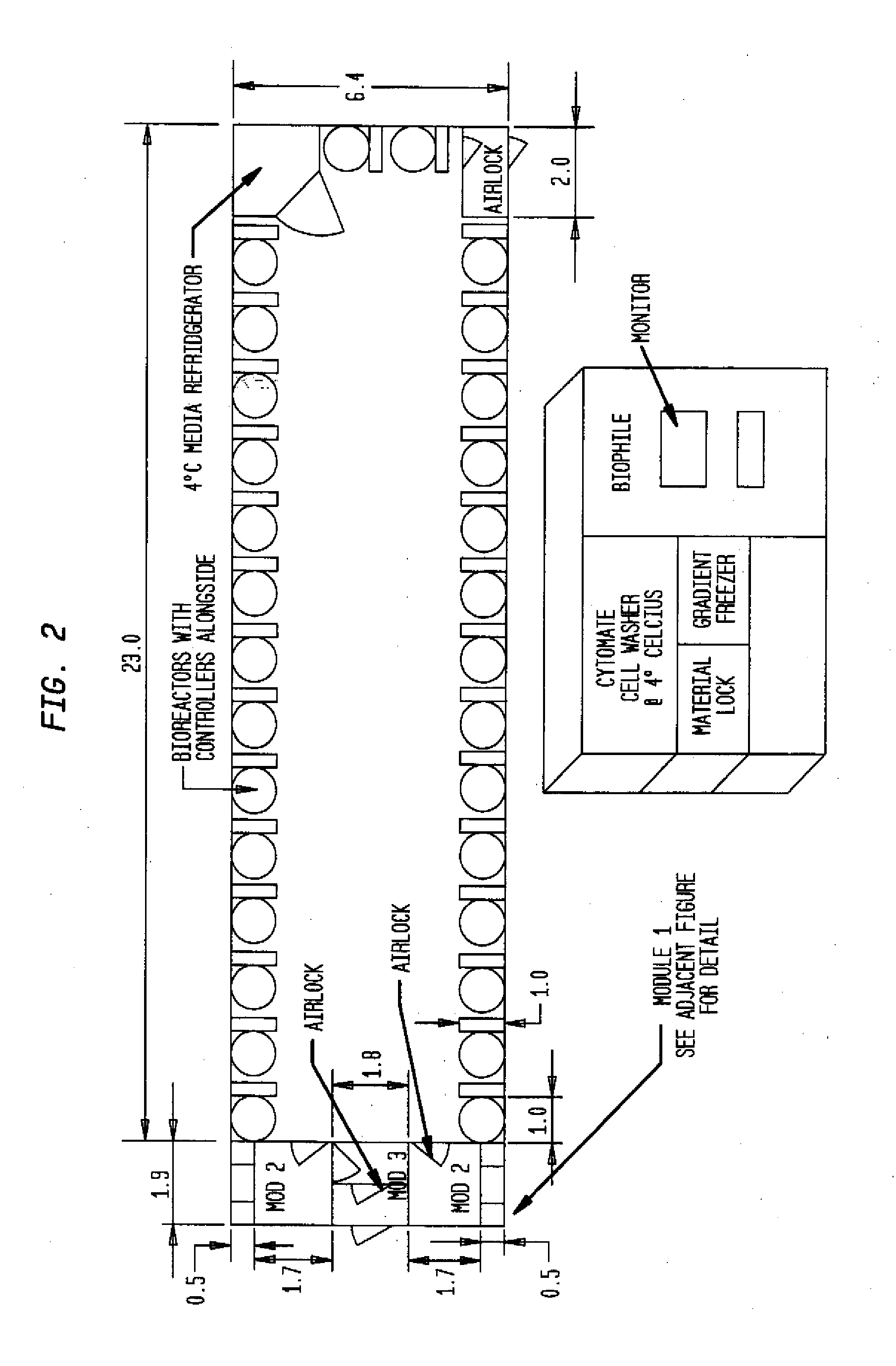
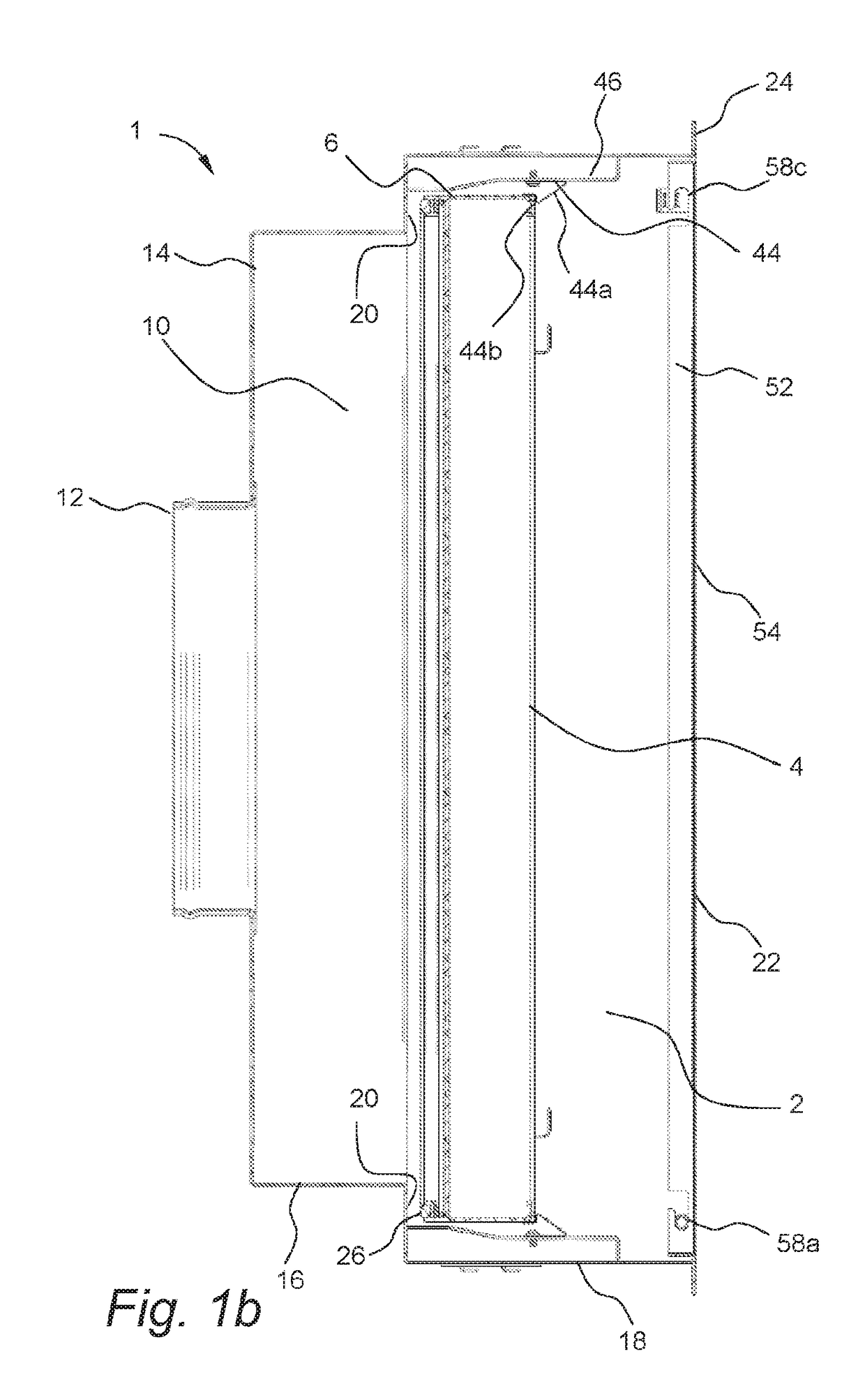
Mobile, modular cleanroom facility
ActiveUS20120077429A1Mechanical apparatusLighting and heating apparatusAir filtrationComputer module
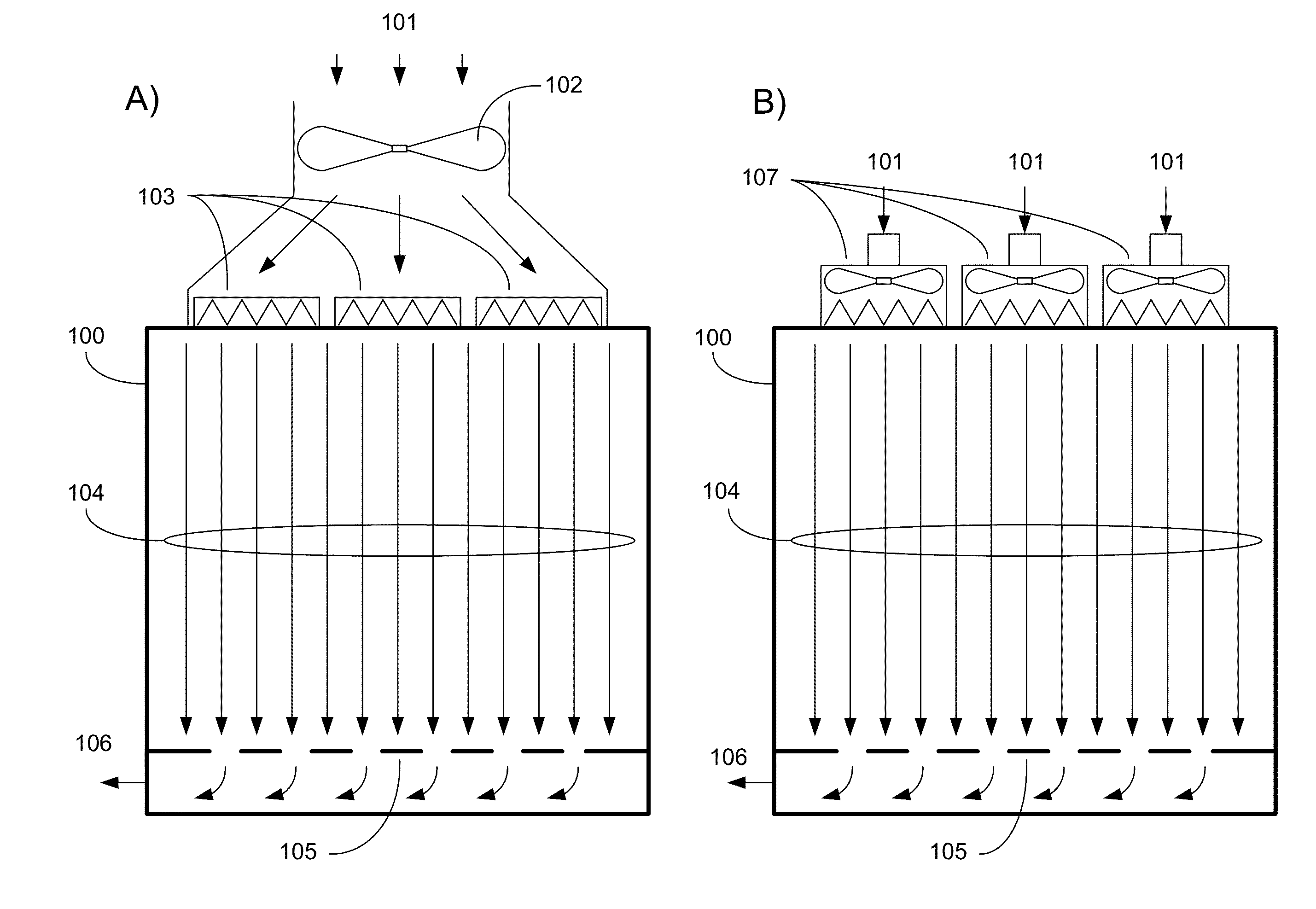
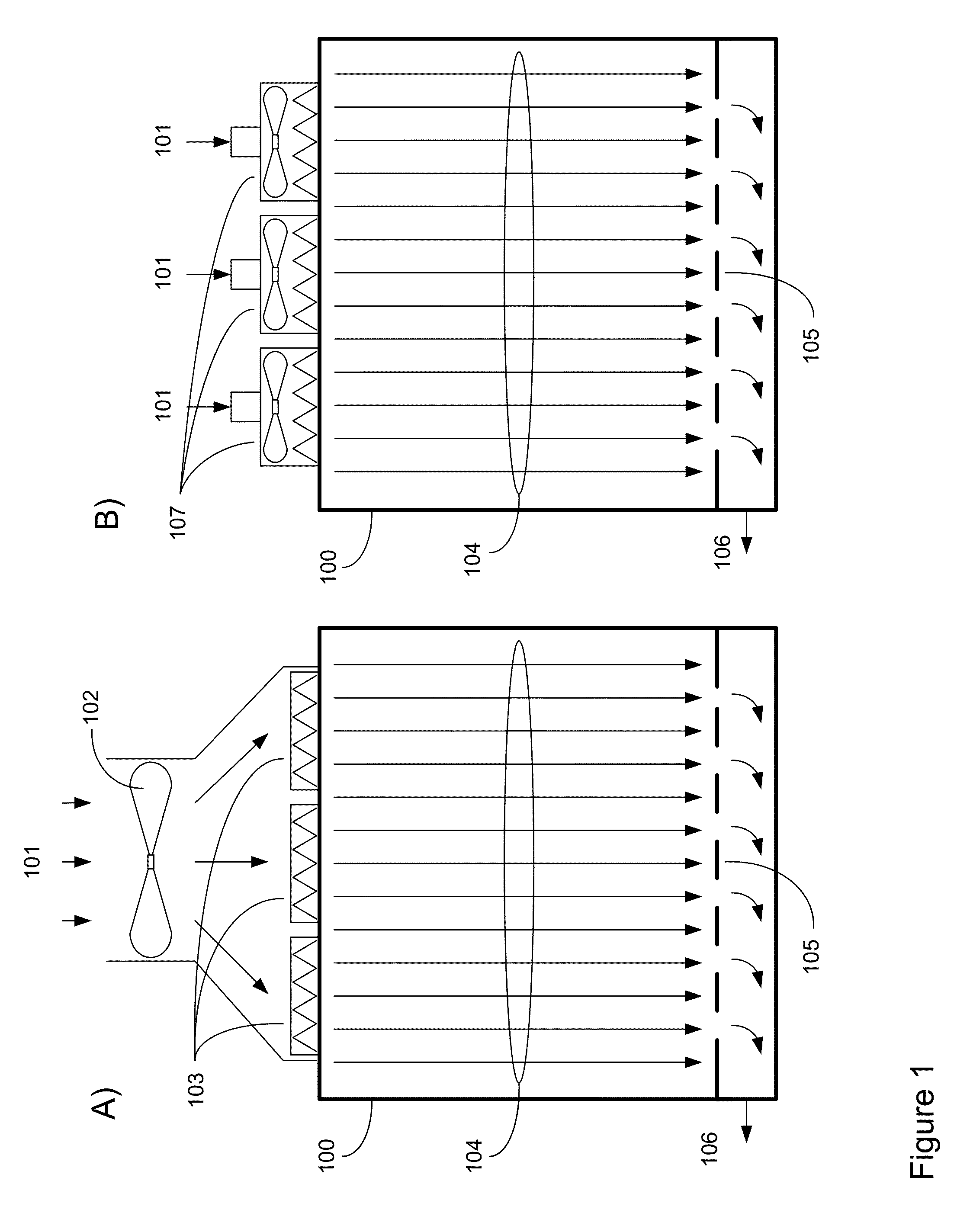
A mobile, modular, cleanroom facility is made from one or more pre-assembled modules, transportable in their pre-assembled form. Each pre-assembled module includes an air filtration system including a ceiling plenum for providing clean air to the interior of the module. The air filtration system provides air cleaned to at least an ISO 8 classification. When the mobile, modular cleanroom facility is made of two or more modules, each of the modules is pre-assembled, and is transportable in its pre-assembled form. Each of the modules also includes an air filtrations system having a ceiling plenum for providing clean air to the interior of the module. Most preferably, the modules are connected by a connection assembly effective for providing a seamless transition from one module to the other while maintaining the appropriate clean air classification in the transition space.
Owner:BIOLOGICS MODULAR
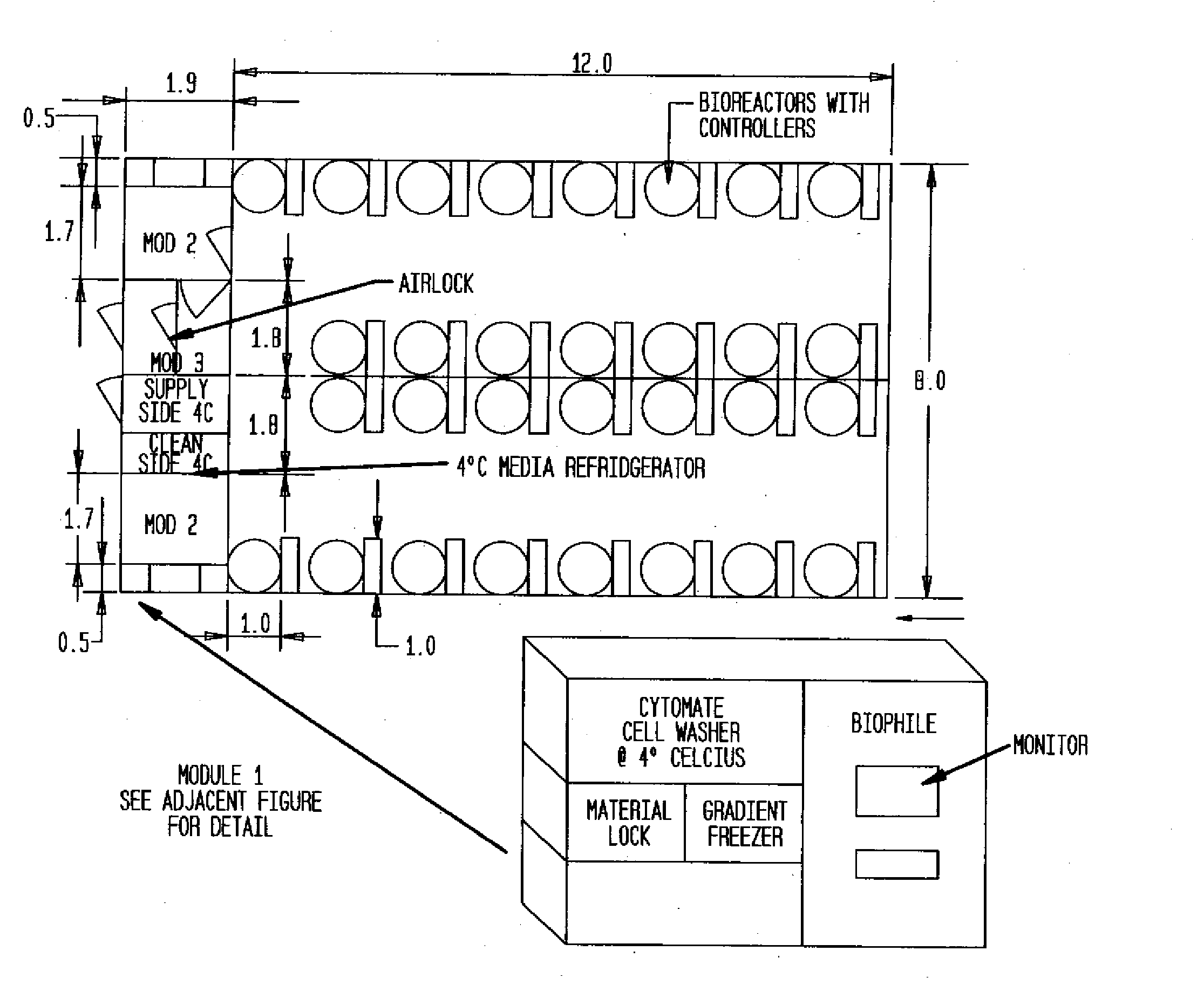
Prevalidated, modular good manufacturing practice-compliant facility
InactiveUS20090305626A1Precious timeShorten the timeApparatus sterilizationDust-free enclosuresInterior spaceGood laboratory practice
The invention is directed to a ready-to-use modular cleanroom and facility, in particular for the production of drugs and biological substances, which is equipped with pre-approved manufacturing equipment cores. The modular cleanroom is implemented in the interior space of a container, such as a standard shipping container, and includes at least one bioreactor station. The modular facility can be installed on-site from pre-approved cleanroom modules without further regulatory approval. The cleanroom and facility comply with FDA-approved good manufacturing practices (GMP) and good laboratory practices (GLP).
Owner:HOPE ERNEST G
Low-cost, user-friendly hardcoating solution, process and coating
InactiveUS6265029B1Excellent abrasion resistanceArticle accumulatePretreated surfacesCoatingsColloidal silicaSilanes
An improved, user-friendly silane / silica sol copolymer hardcoating composition for protecting optical plastic and other substrates (including wood, metals, glass, plastics and most coated articles) from scratching is able to be manufactured at lower costs and to provide much-improved ease of use in transportation, storage, dipbath (or other coating tank) stability, and blush resistance in ordinary cleanroom atmospheres. The silane / silica sol copolymer is formed as a direct reaction product of an acidic silica sol and monomethyltrialkoxysilane, preferably substantially monomethyltriethoxysilane, in ratios of 30:70 to 70:30, most preferably about 40:60. A tail solvent aids blush resistance, reduces internal stress and permits adhesion to unprimed polycarbonate. Optionally, colloidal silica sol dissolved in water-miscible solvent may be reacted in a second stage with acidic aqueous colloidal silica sol earlier silanized with monomethyltrialkoxysilane.
Owner:LEWIS WILLIAM
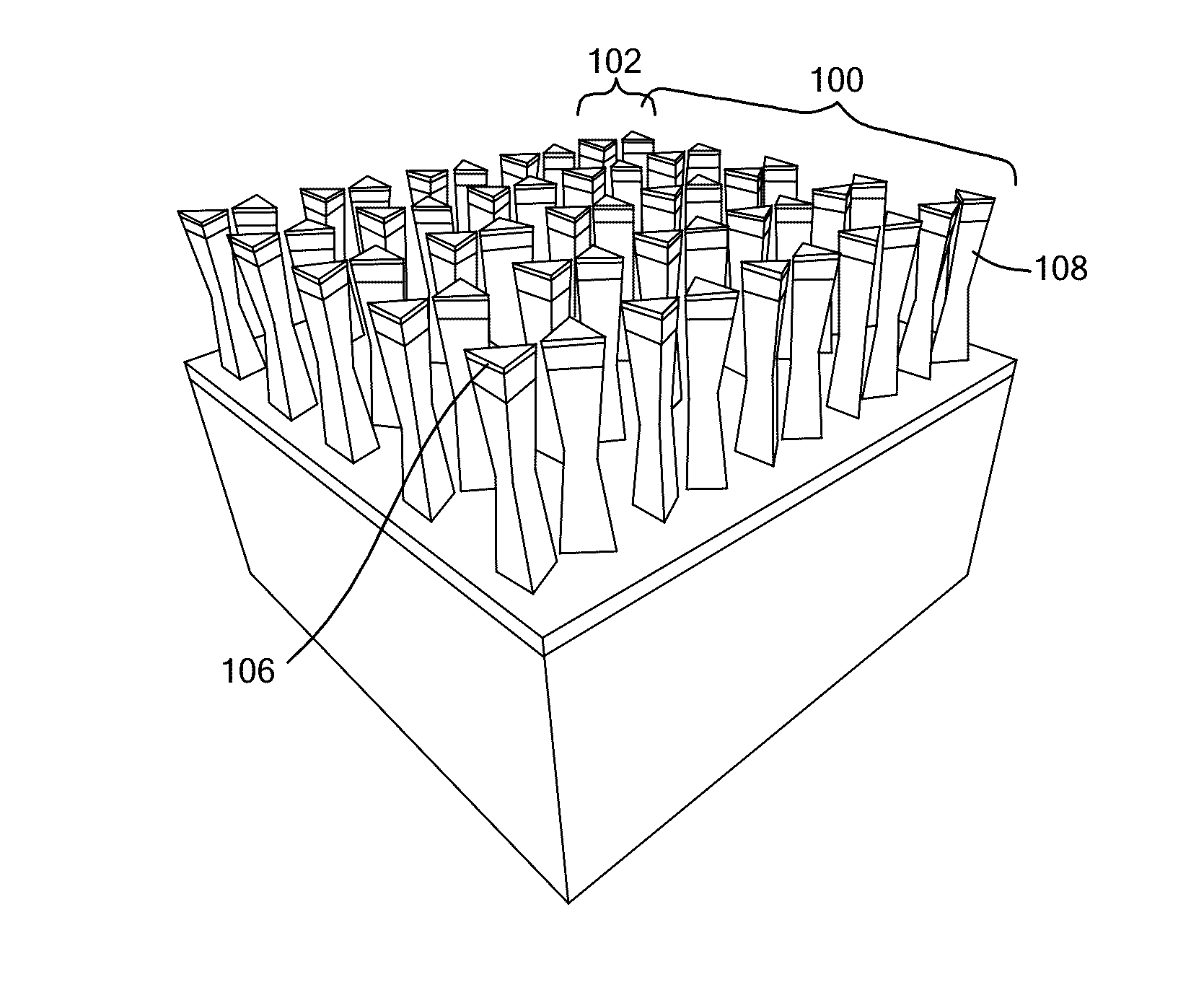
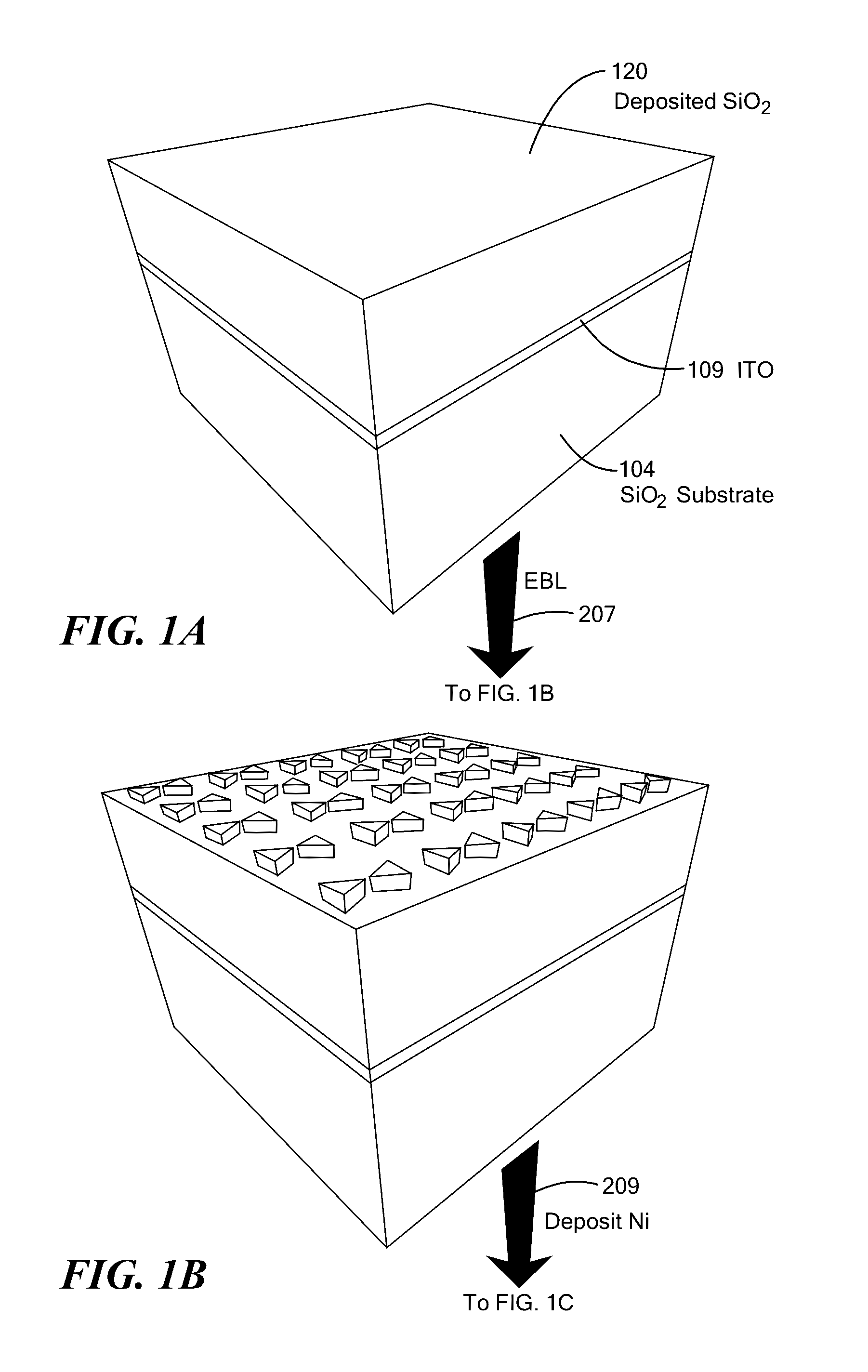
Bowtie Nanoantennas and Methods of Using the Same
A pillar-nanoantenna array structure is fabricated with a substrate to which pairs of pillars are coupled, where the pillars are characterized either by a thermal conductance less than 0.1 μW / deg or by transparency and a height exceeding thickness by at least a factor of two. Metallic caps atop a neighboring pair of pillars are separated by no more than 50 nm. An image-capture structure may be formed by modifying reflectance of a portion of the structure by heating of the portion by electromagnetic radiation. The array may be plastically deformed by raster scanning an electron beam across the array, exciting plasmon modes in the conducting particles thereby inducing a gradient force between neighboring conducting particles, and deforming neighboring pillars in such a manner as to vary the spacing separating neighboring conducting particles. A technique of plasmon-assisted etching provides for fabricating specified planar pattern of metal outside a cleanroom environment.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Apparatus for storing and moving a cassette
InactiveUS20010043849A1Semiconductor/solid-state device manufacturingCharge manipulationDocking stationMagnetic tape
Owner:PERLOV ILYA +2
System for Managing a Cleanroom Environment
InactiveUS20150056909A1Low costReduce energy useMechanical apparatusSpace heating and ventilation safety systemsHEPADifferential pressure
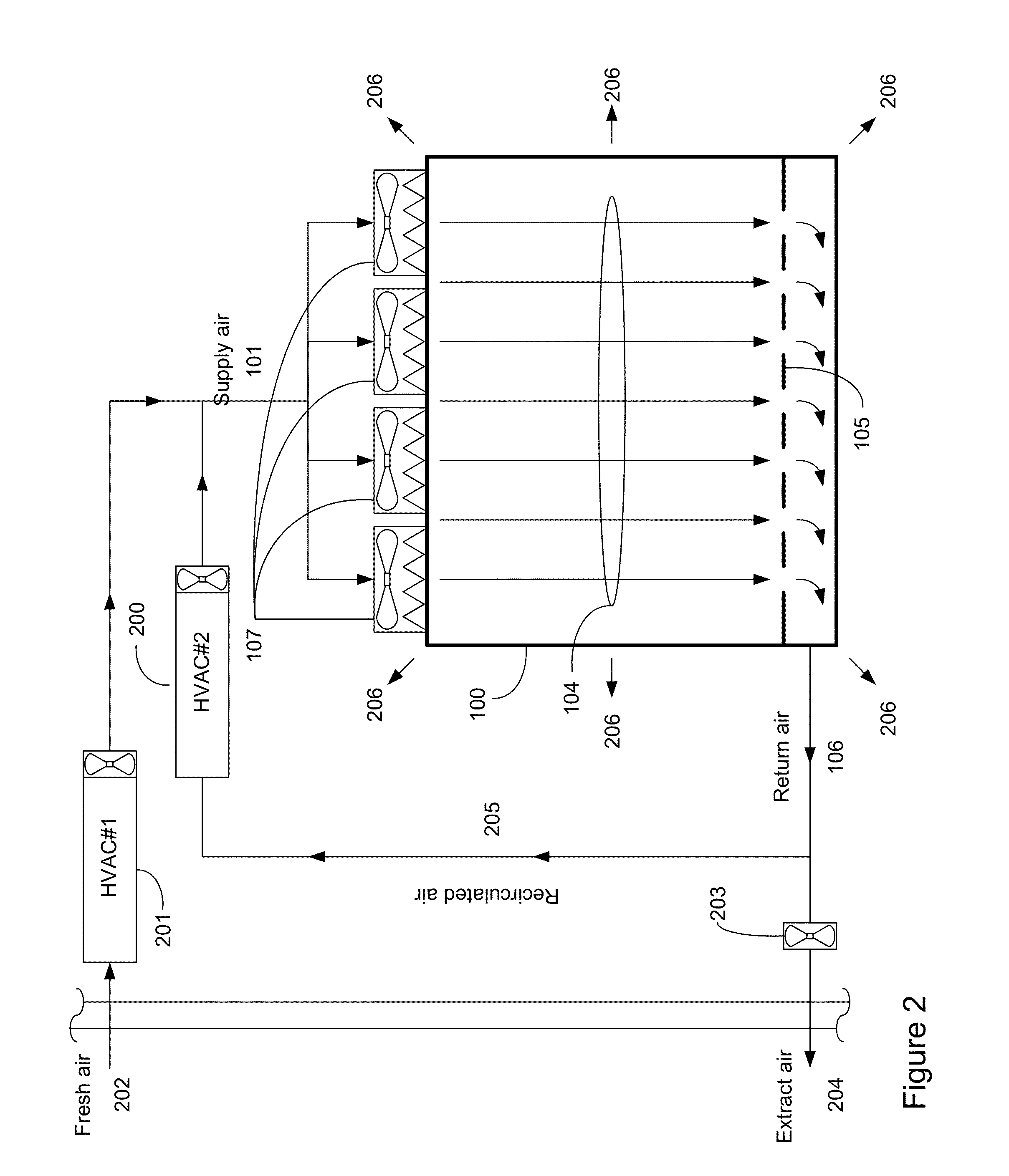
A system for managing cleanroom resources by providing a number of critical features in an integrated, low-cost package is described. The critical features include monitoring and recording cleanroom environmental conditions such as temperature, humidity and room differential pressure, notifying users of alarm situations when cleanroom environmental conditions fall outside predetermined limits, and reducing cleanroom energy usage by turning off HEPA filter fan units (FFUs) and cleanroom lights when they are not needed.
Owner:CHIEN ANTHONY
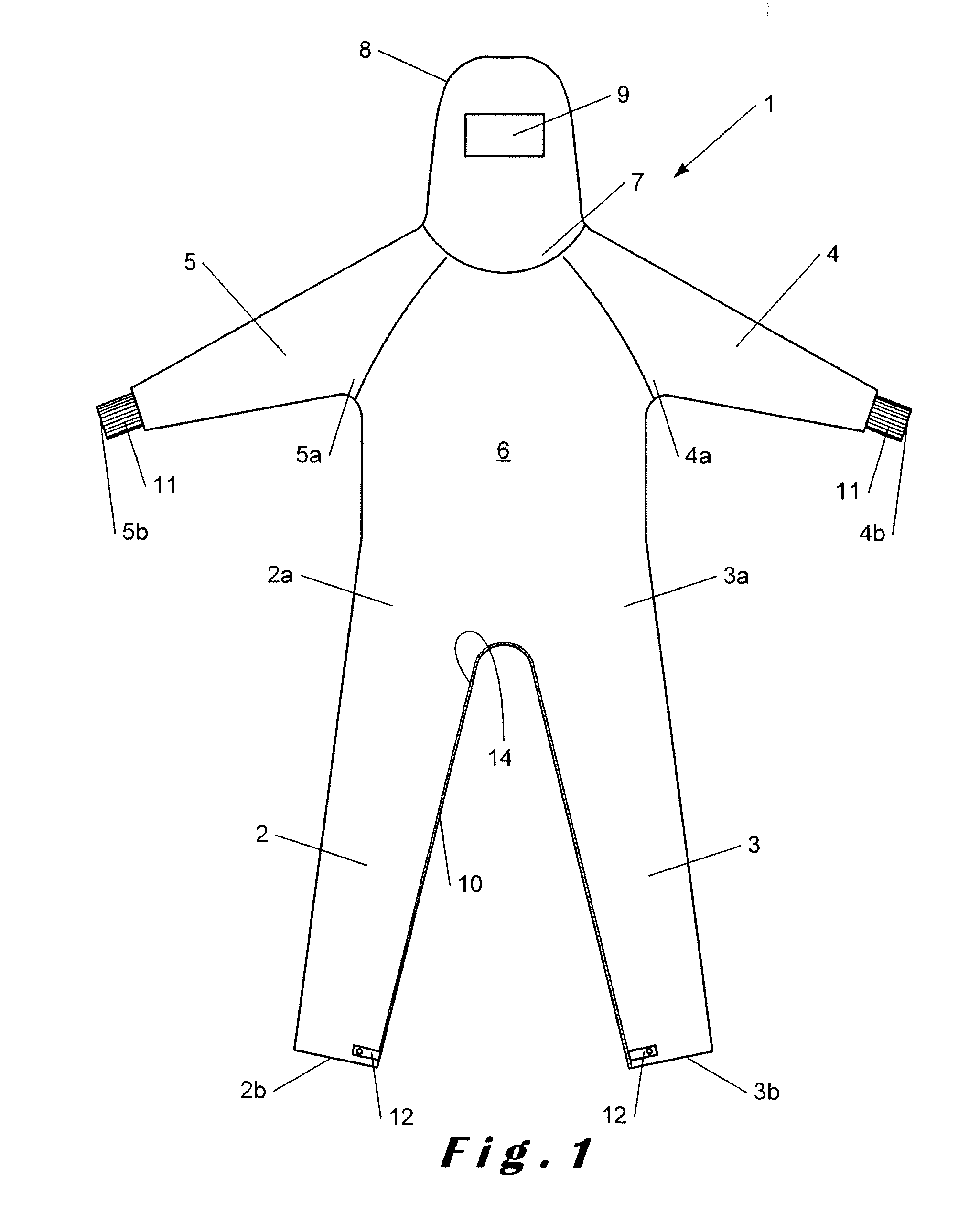
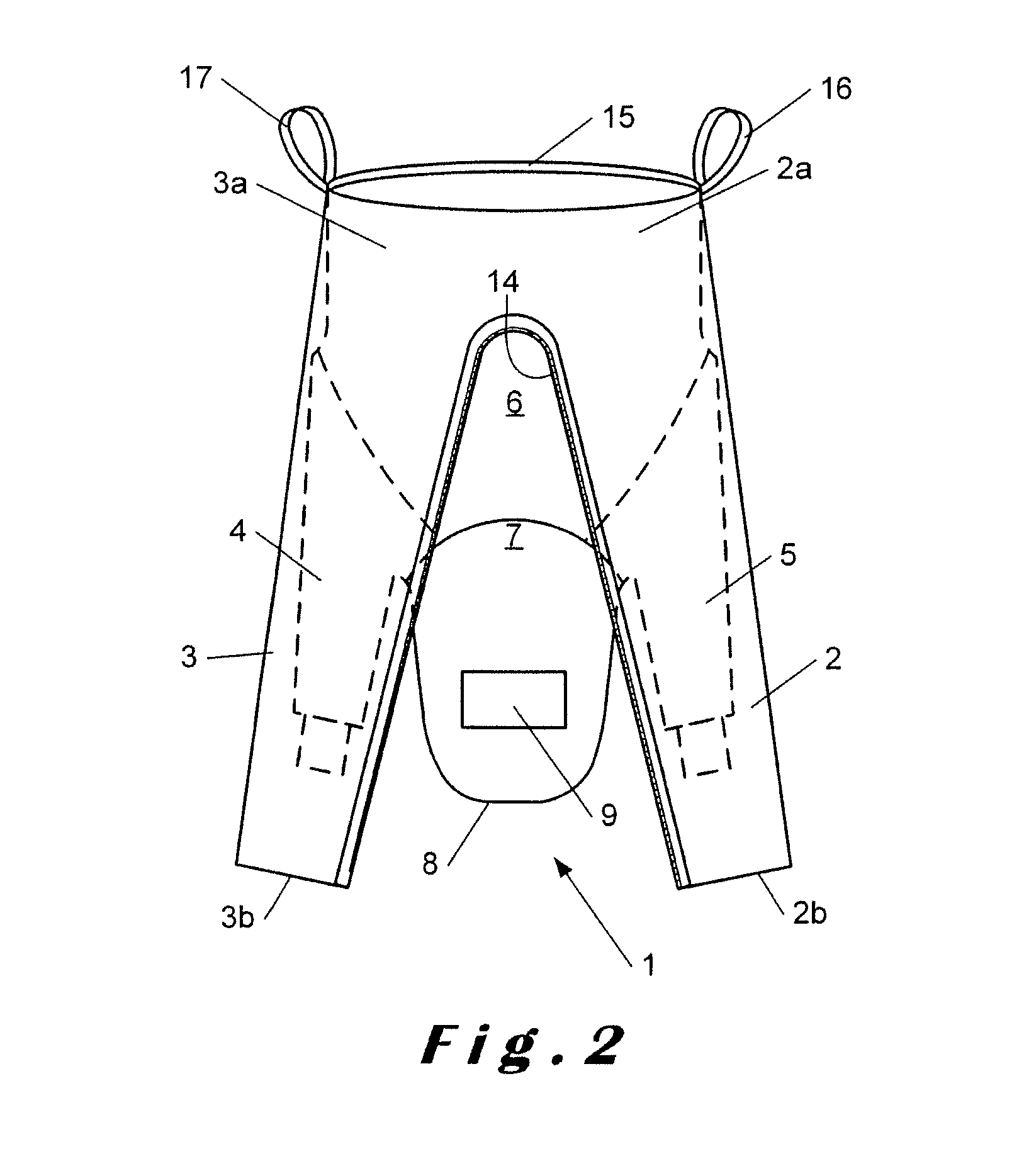
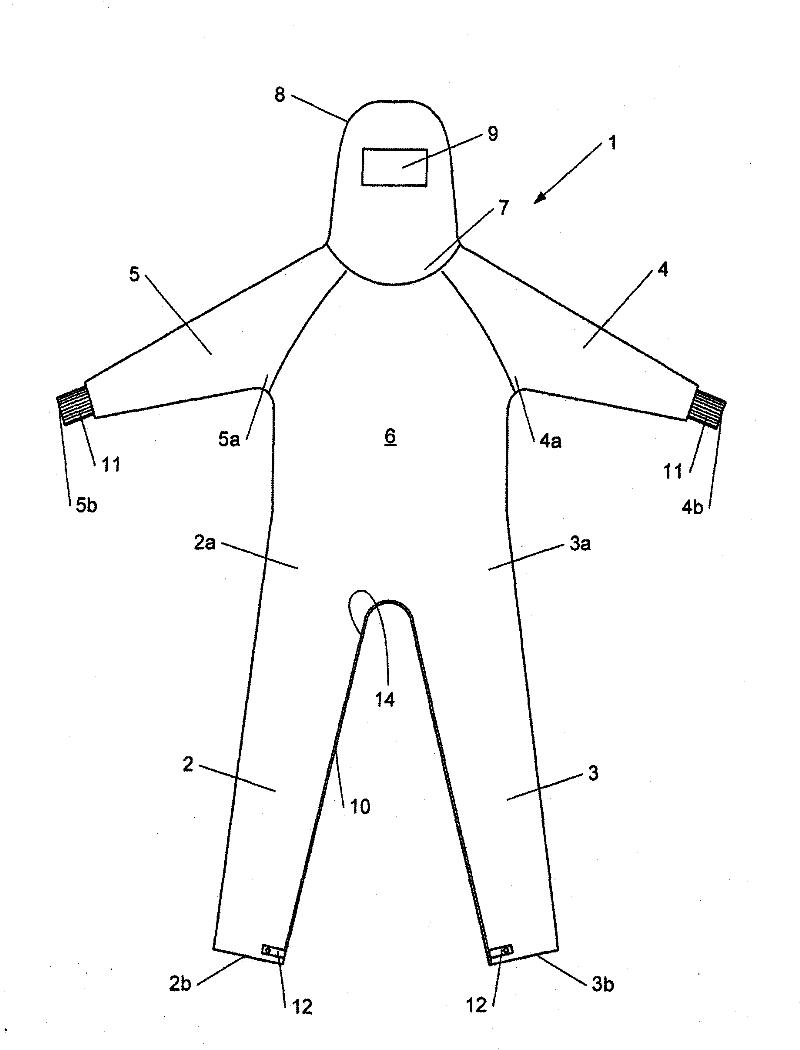
Overalls for cleanroom and the like
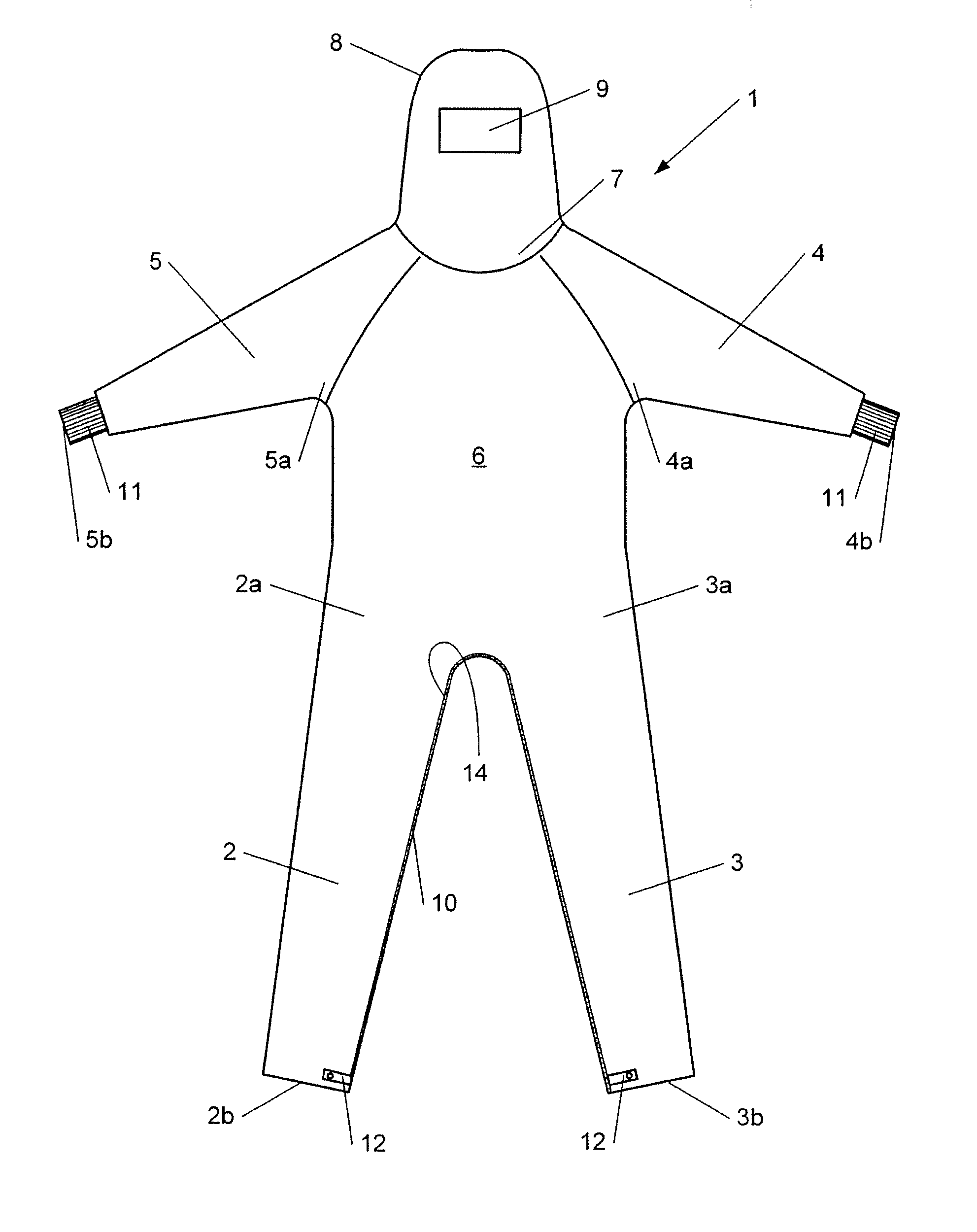
InactiveUS20150096099A1Avoid mistakesChemical protectionGarment special featuresEngineeringCleanroom
Owner:SCALDIS SAINT MARTIN
Low-cost, user-friendly hardcoating solution, process and coating
An improved, user-friendly silane / silica sol copolymer hardcoating composition for protecting optical plastic and other substrates (including wood, metals, glass, plastics and most coated articles) from scratching is able to be manufactured at lower costs and to provide much-improved ease of use in transportation, storage, dipbath (or other coating tank) stability, and blush resistance in ordinary cleanroom atmospheres. The silane / silica sol copolymer is formed as a direct reaction product of an acidic silica sol and monomethyltrialkoxysilane, preferably substantially monomethyltriethoxysilane, in ratios of 30:70 to 70:30, most preferably about 40:60. A tail solvent aids blush resistance, reduces internal stress and permits adhesion to unprimed polycarbonate. Optionally, colloidal silica sol dissolved in water-miscible solvent may be reacted in a second stage with acidic aqueous colloidal silica sol earlier silanized with monomethyltrialkoxysilane.
Owner:LEWIS WILLIAM
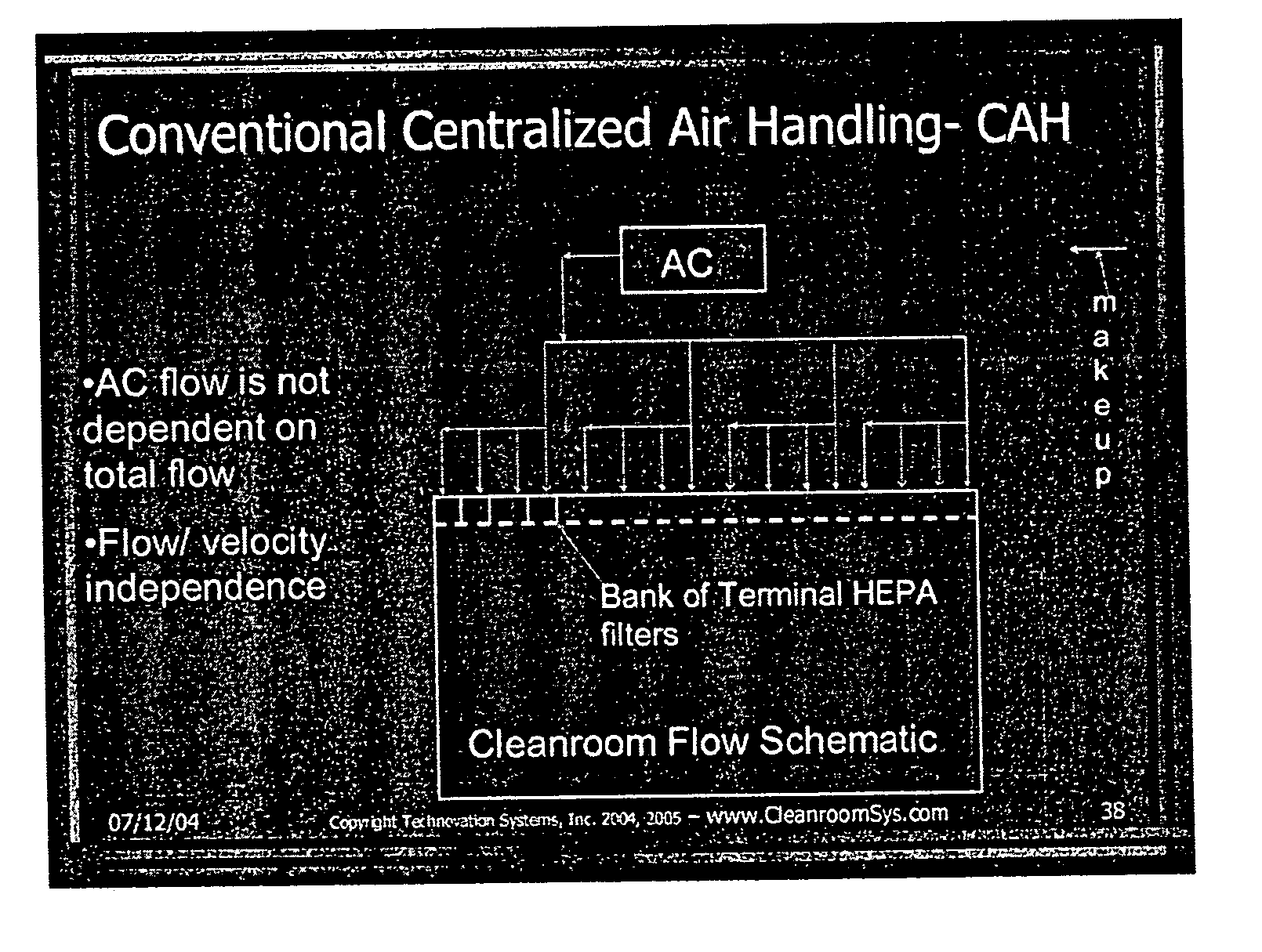
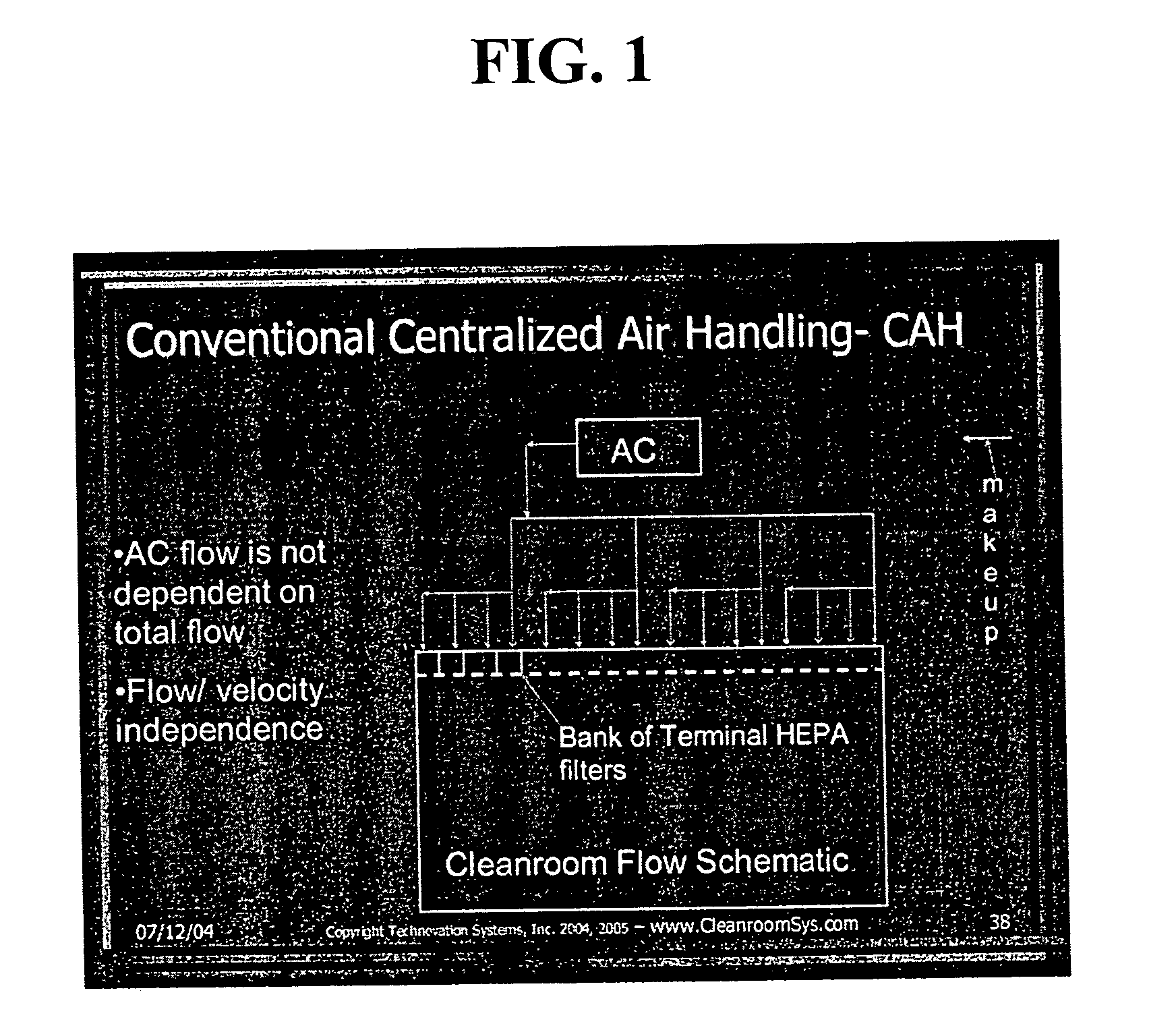
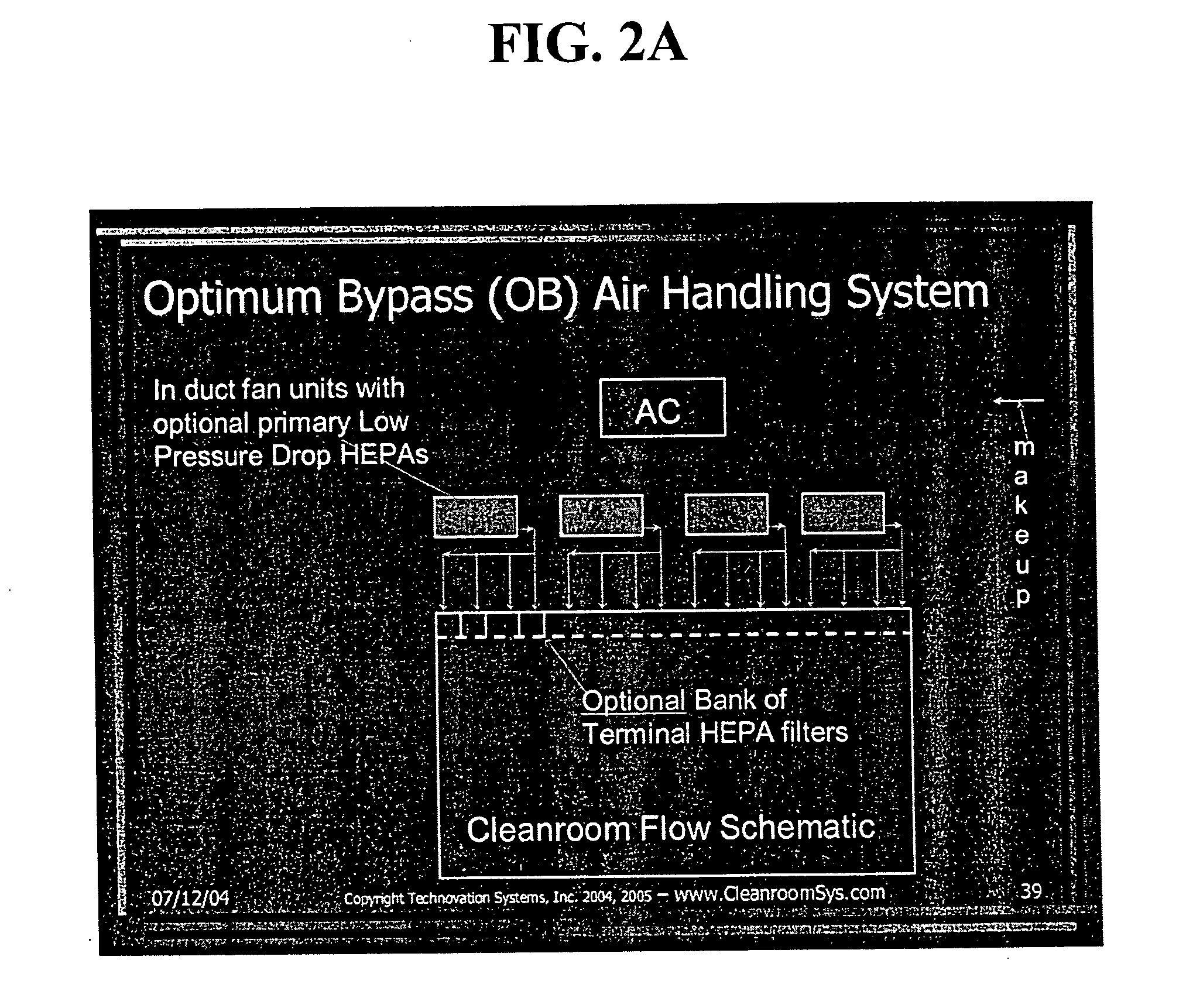
Energy efficient air handling system for cleanrooms
InactiveUS20070089854A1Low costEliminate useMechanical apparatusMilk preservationEngineeringAir change
A refrigeration based air handling system design process for significant energy and cost savings in cleanroom and other applications requiring large air change rates is presented. The process utilizes a by pass around the air conditioning system, the ratio of bypass to air conditioning flow being such that minimal or no reheat of the air is required for applications having relative humidity (RH) control requirements and with RH control being achieved via cooling. If dehumidification is achieved by adsorptive processes, then the by pass ratio is varied so as to minimize cooling of the heated dry air. In other non relative humidity control applications the bypass is varied to minimize the air conditioning flow, thereby decreasing cost, but by using optimum cooling coil velocities in a manner such that system energy for airflow is minimized. The energy and cost savings achieved by this process vary between 65% to 15% depending on the Class of the cleanroom and / or on the number of air changes per hour required.
Owner:JAISINGHANI RAJAN A
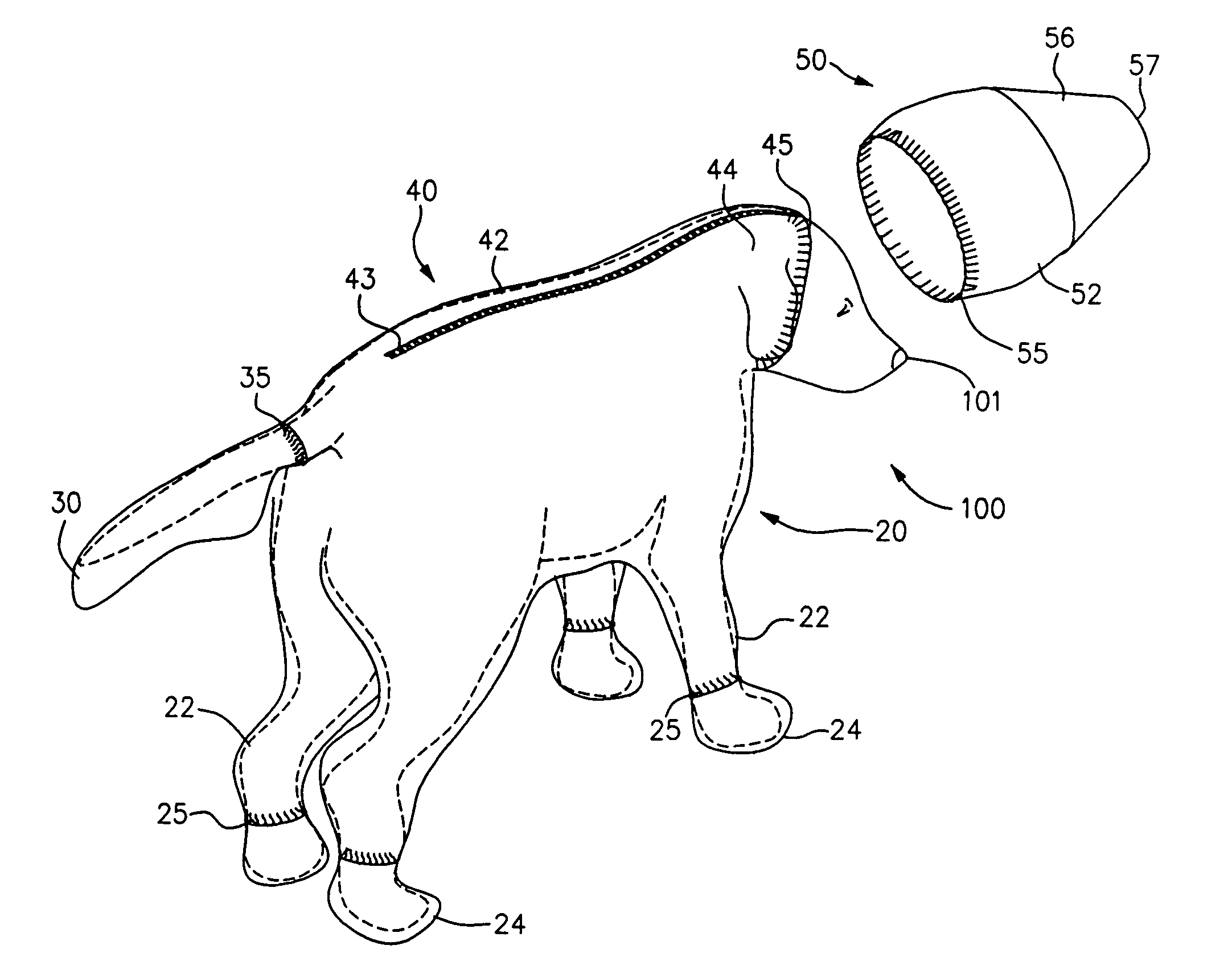
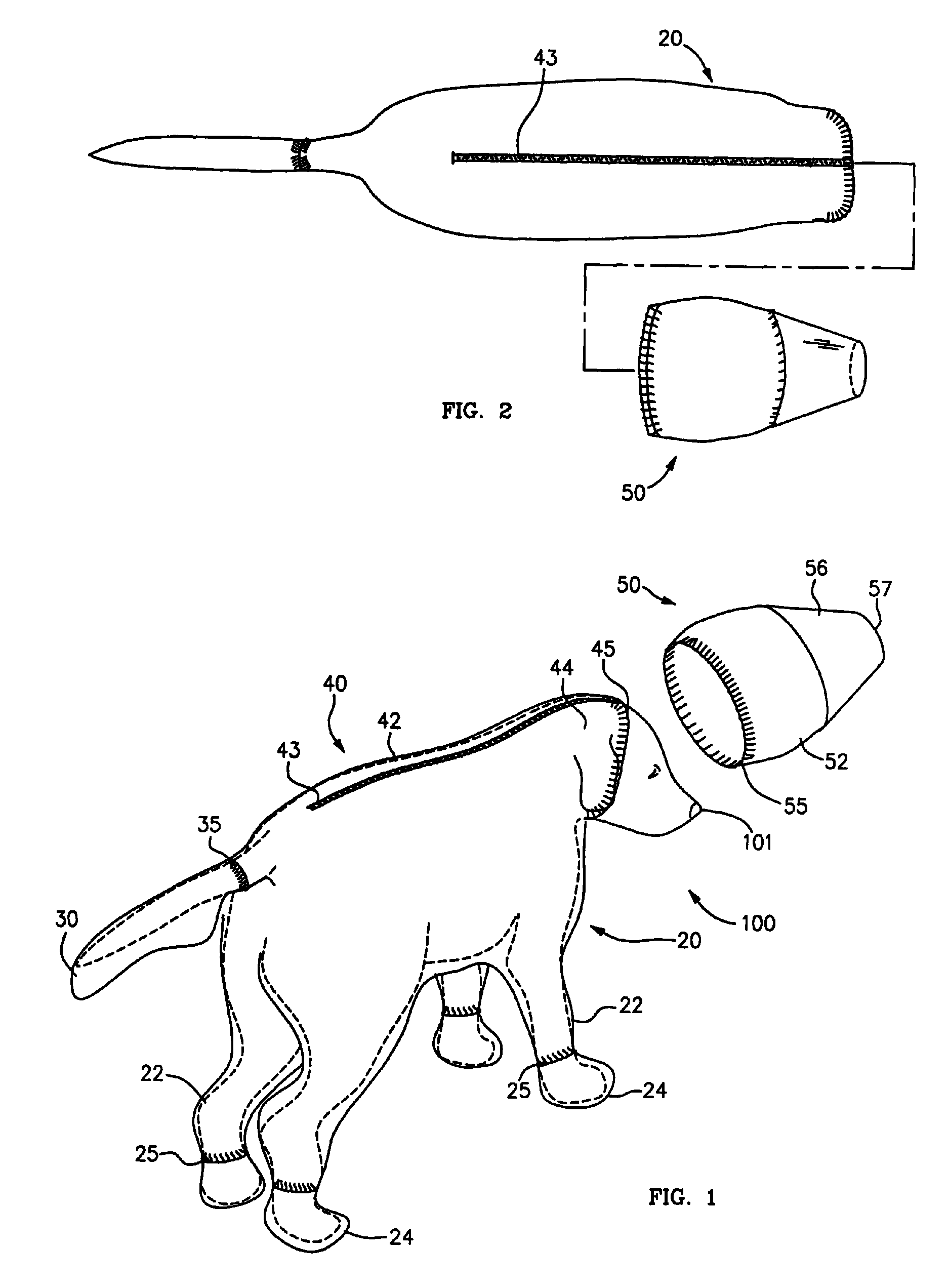
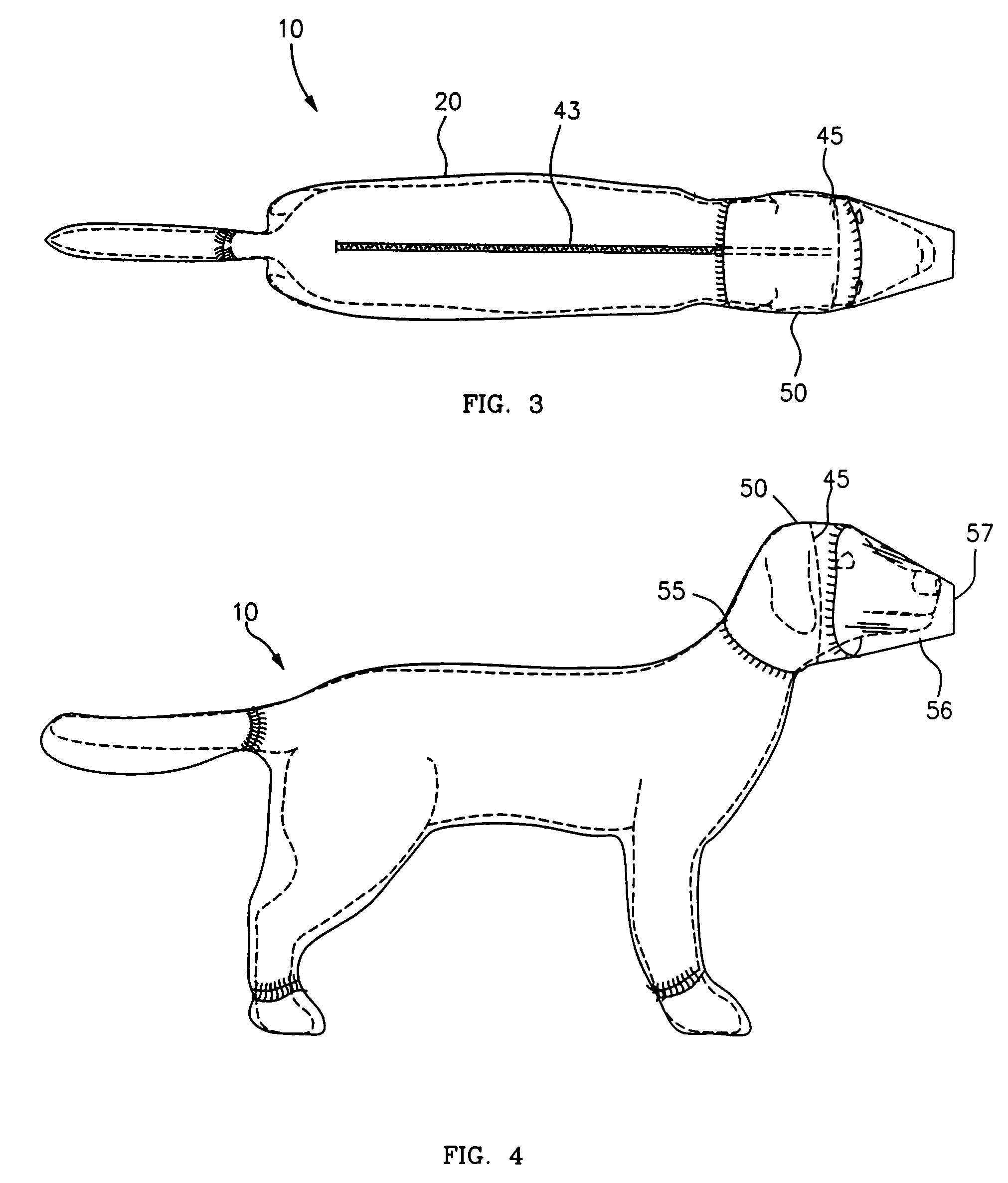
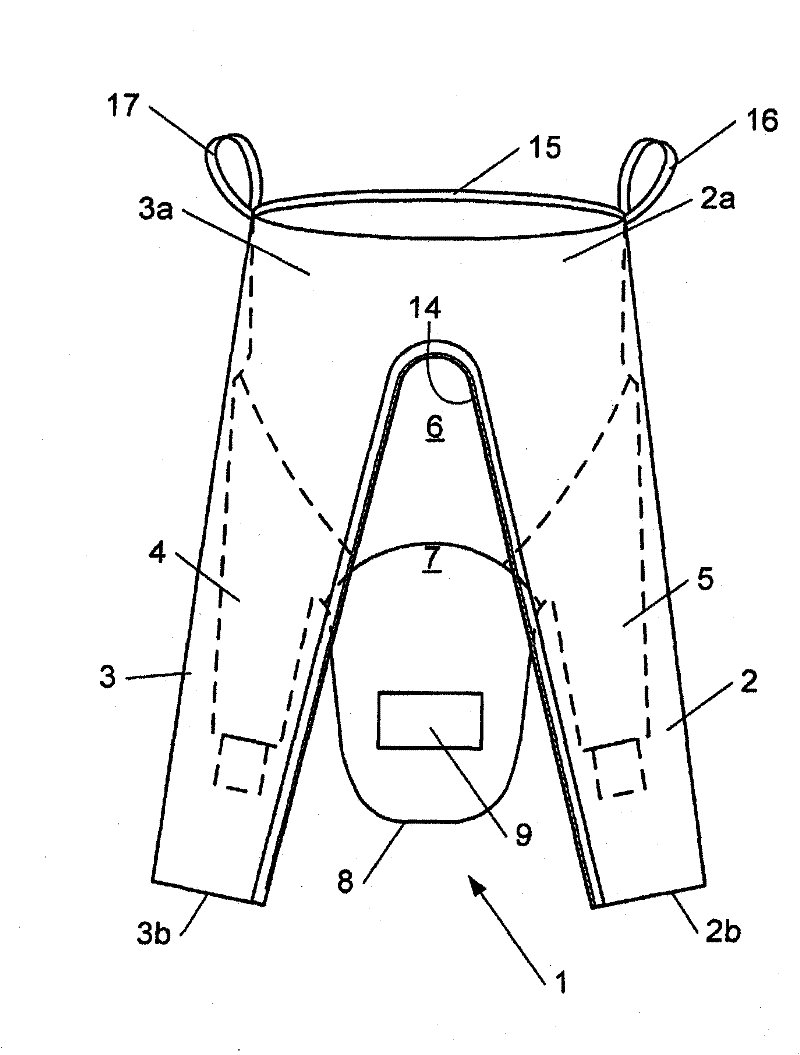
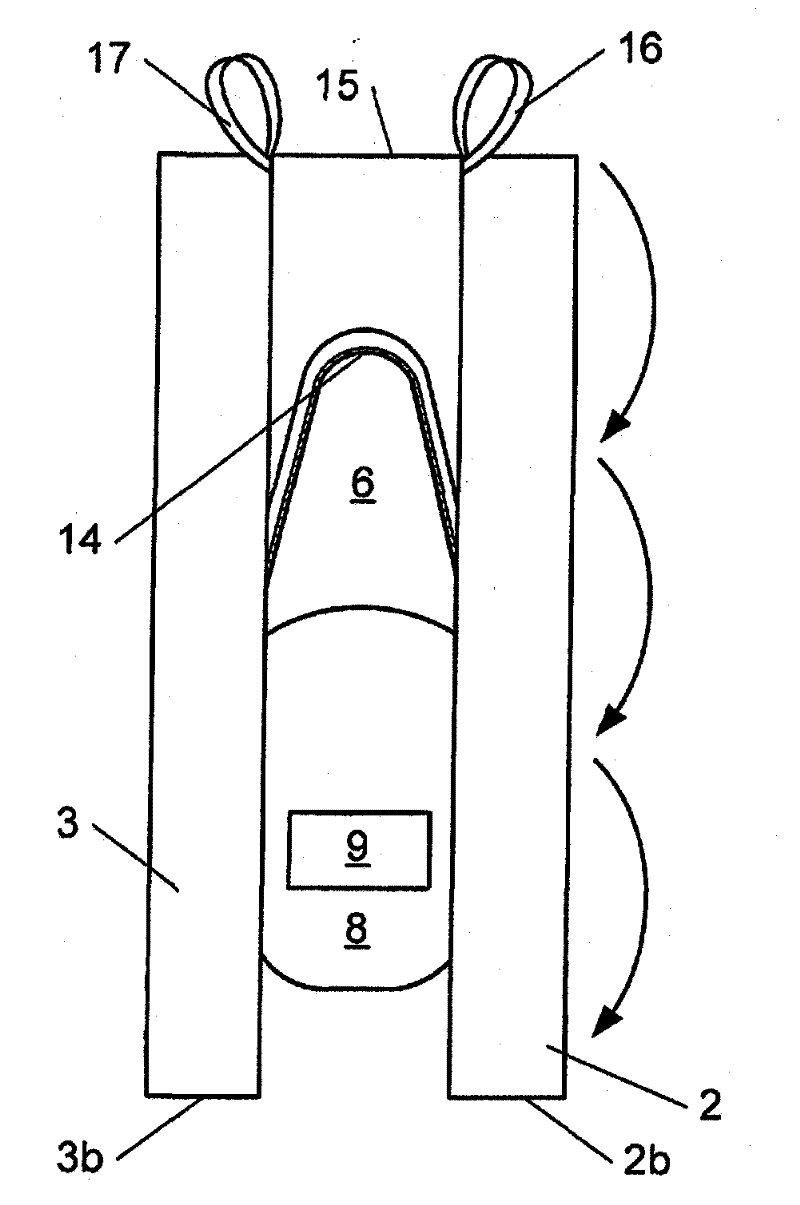
Canine cleanroom suit
Protective suit for a dog allows a dog to work in a cleanroom or other controlled environment. Suit 10 includes particle-blocking coverall 20 and hood 50. Hood 50 includes transparent face shield 52 to cover dog's eyes and snout. Face shield 52 is open at the end to allow air and odors to reach dog's nose unimpeded. Face shield 52 extends slightly beyond dog's nostrils to prevent dog from contacting hazardous chemicals or pathogens. Coverall 20 includes fitted sleeves 22 with integral boots 24; also a tail pouch 30 to keep tail separate and visible.
Owner:PRILL BRADLEY A
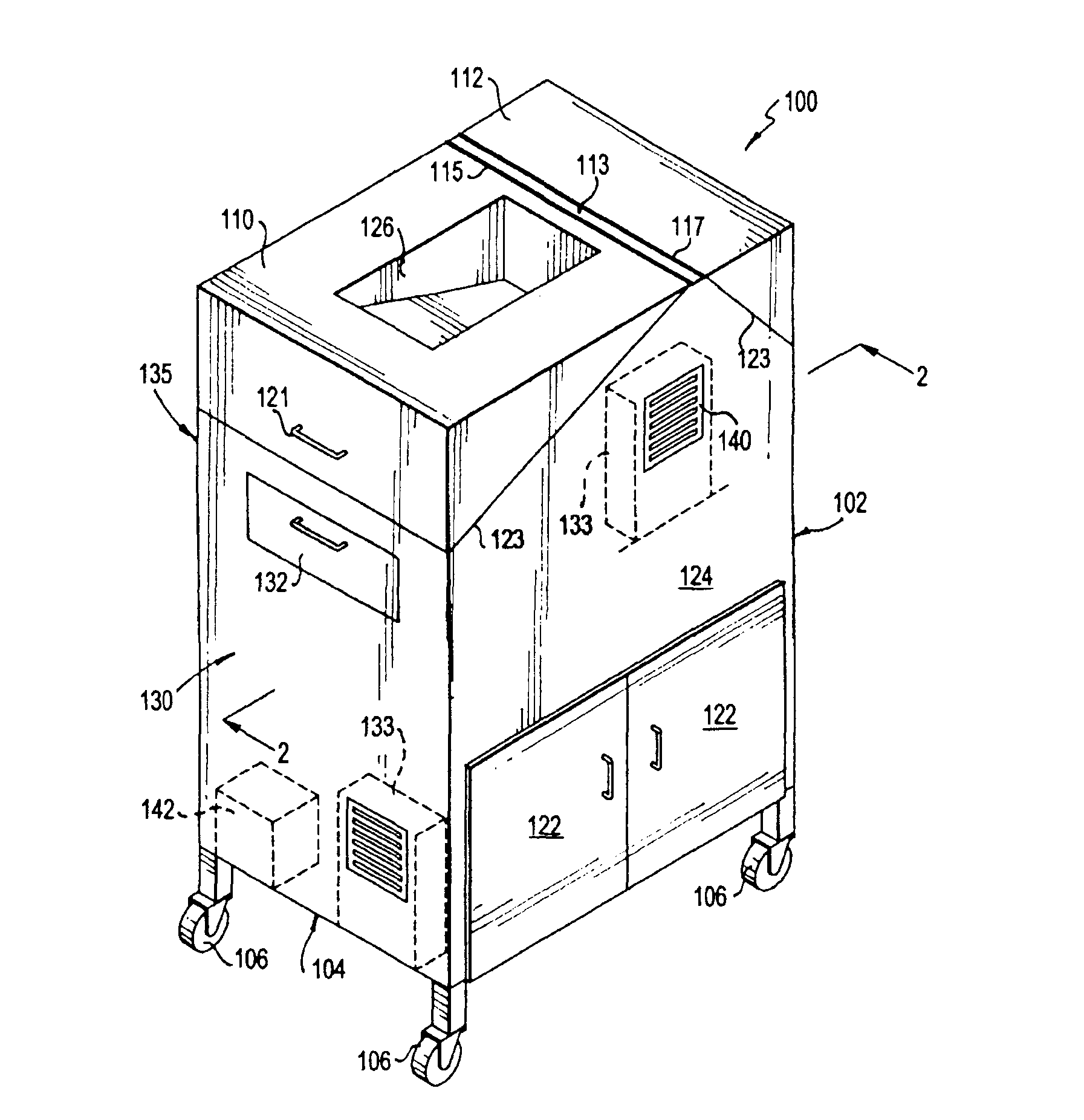
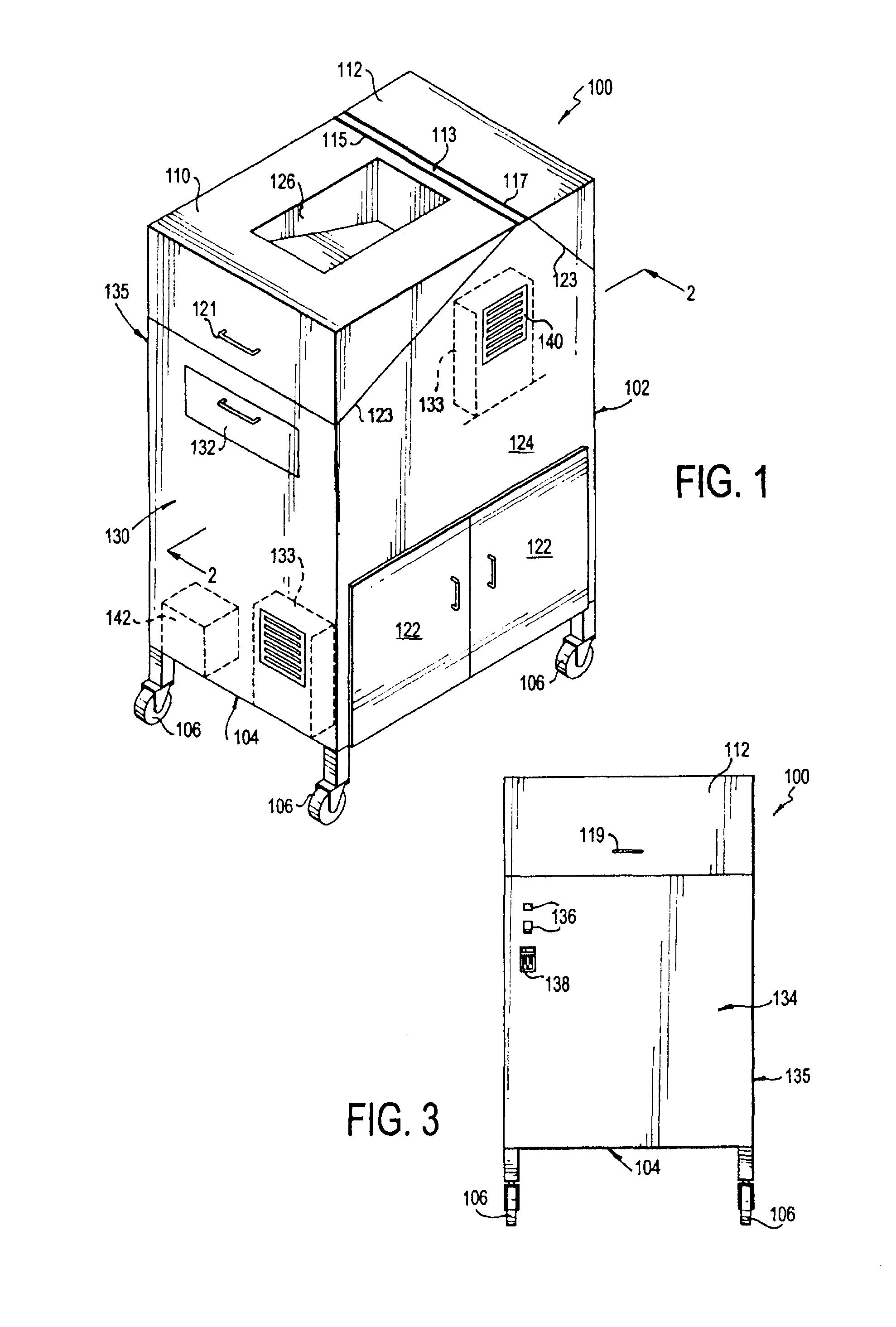
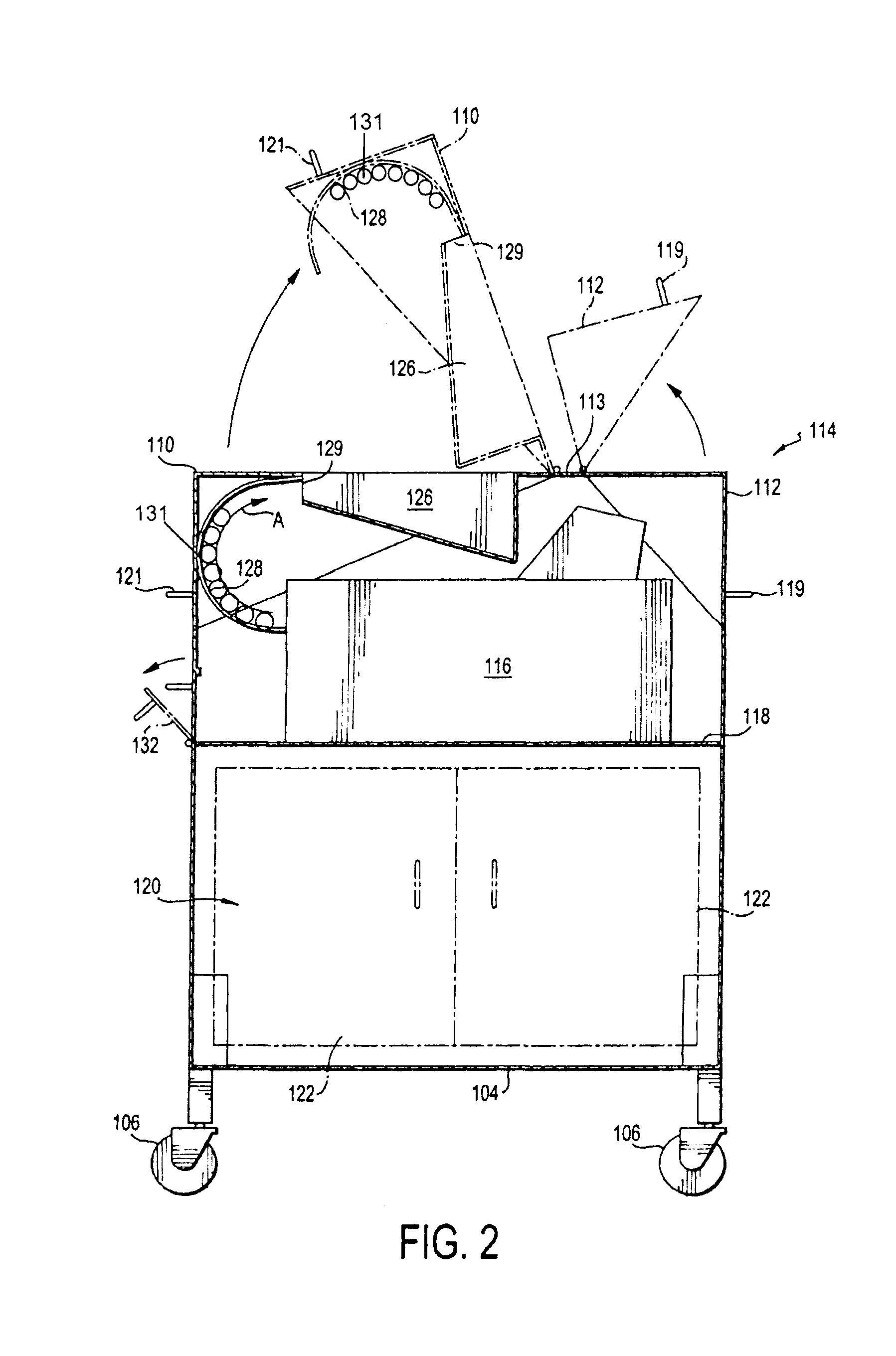
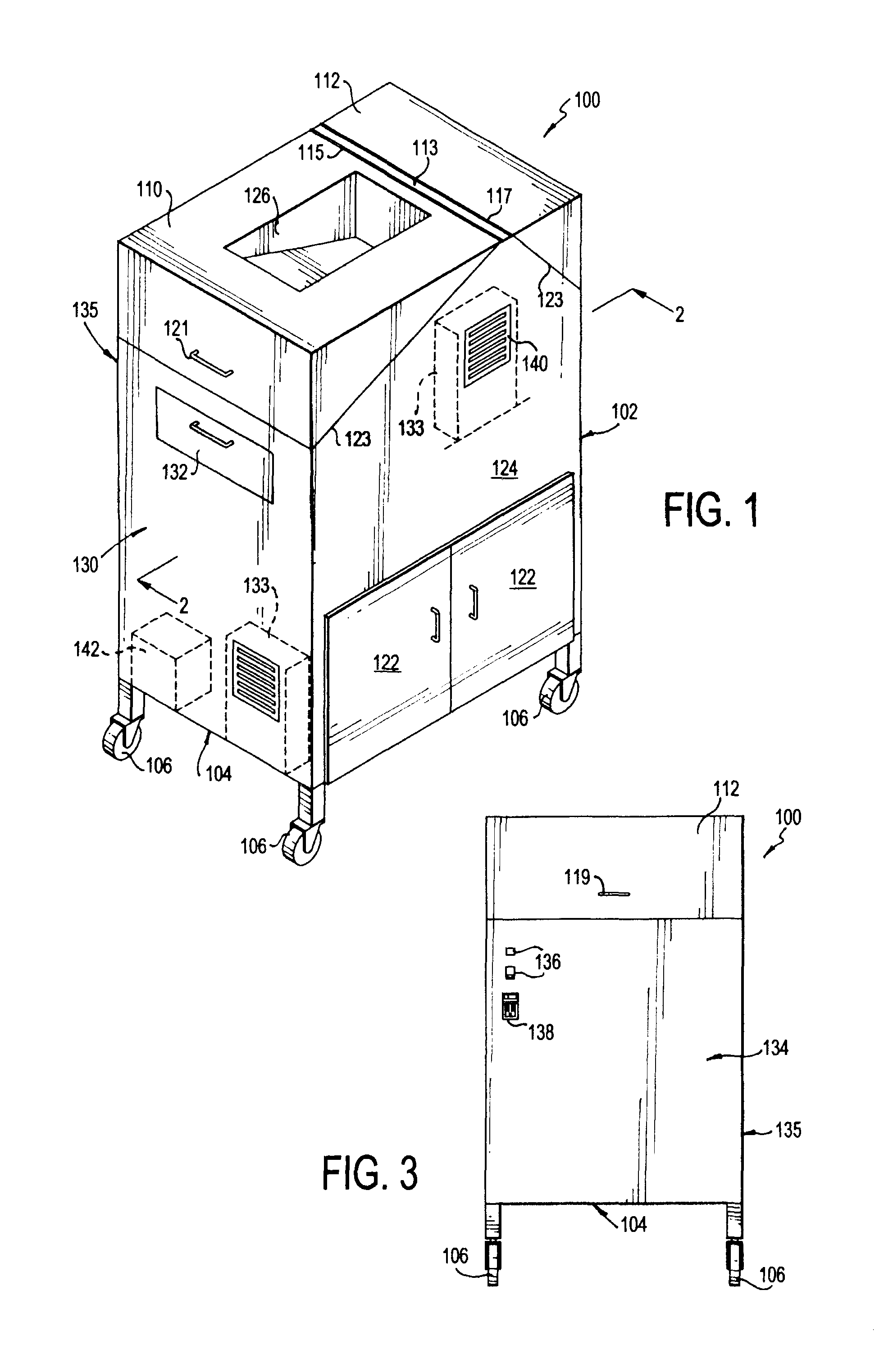
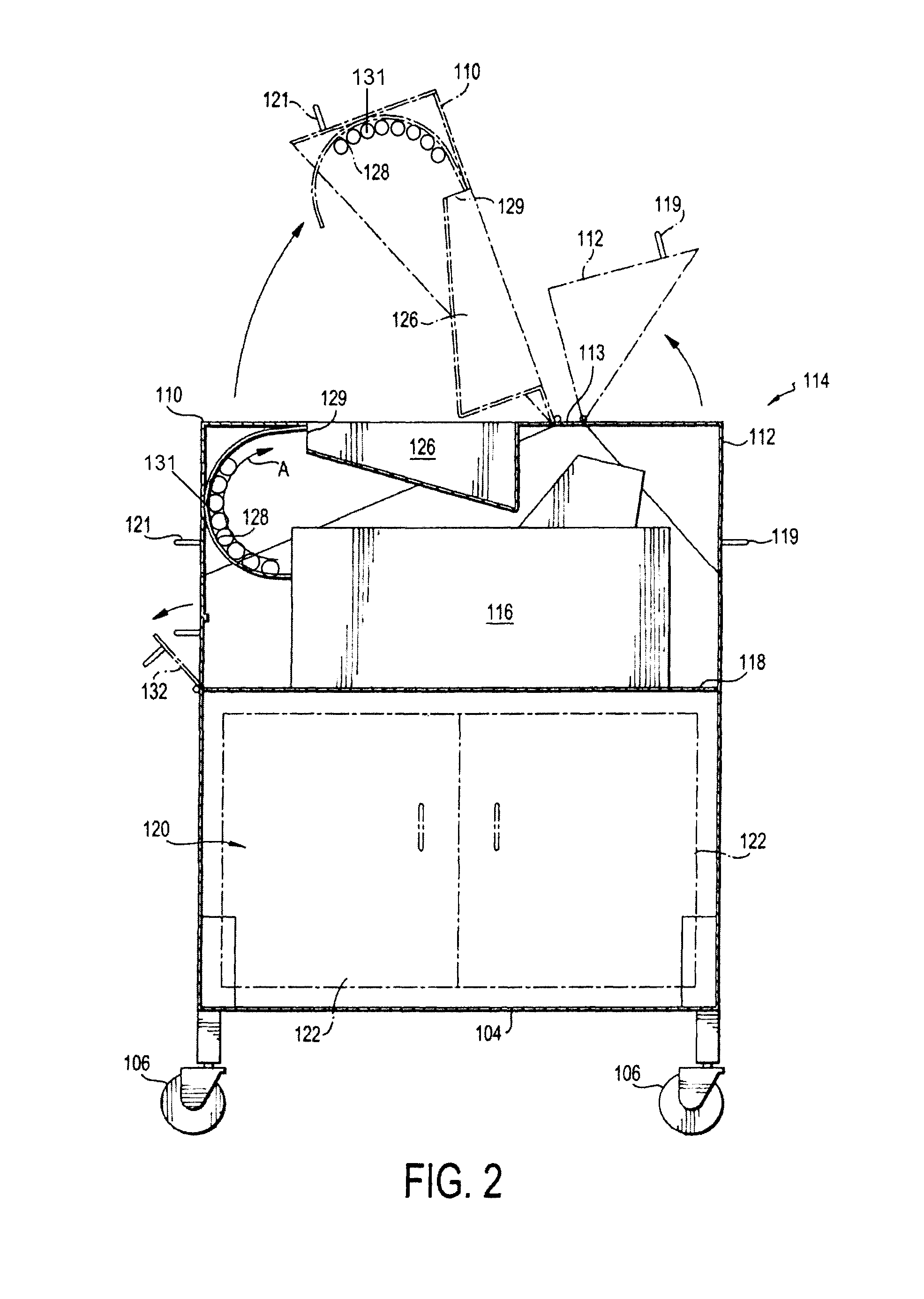
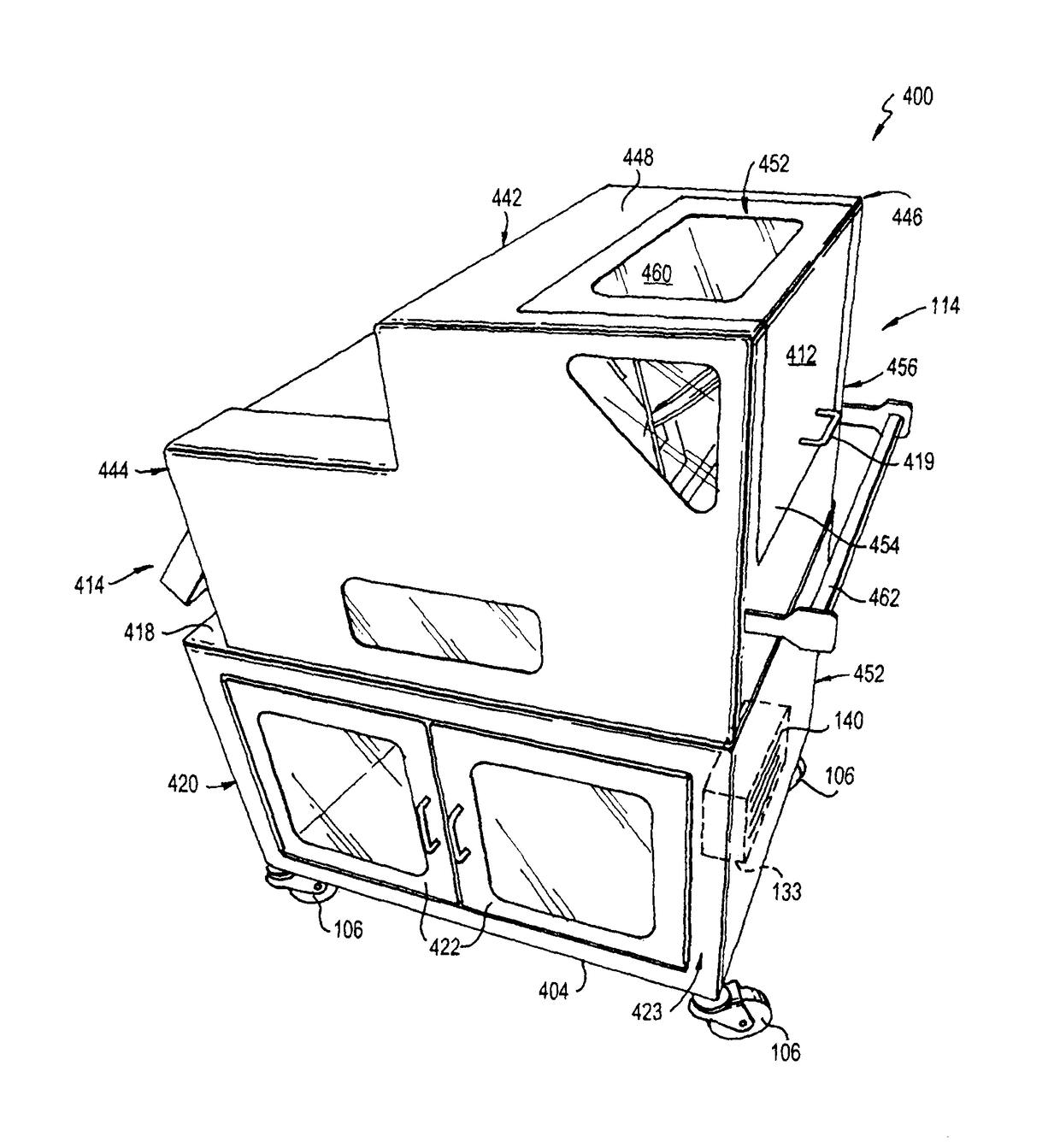
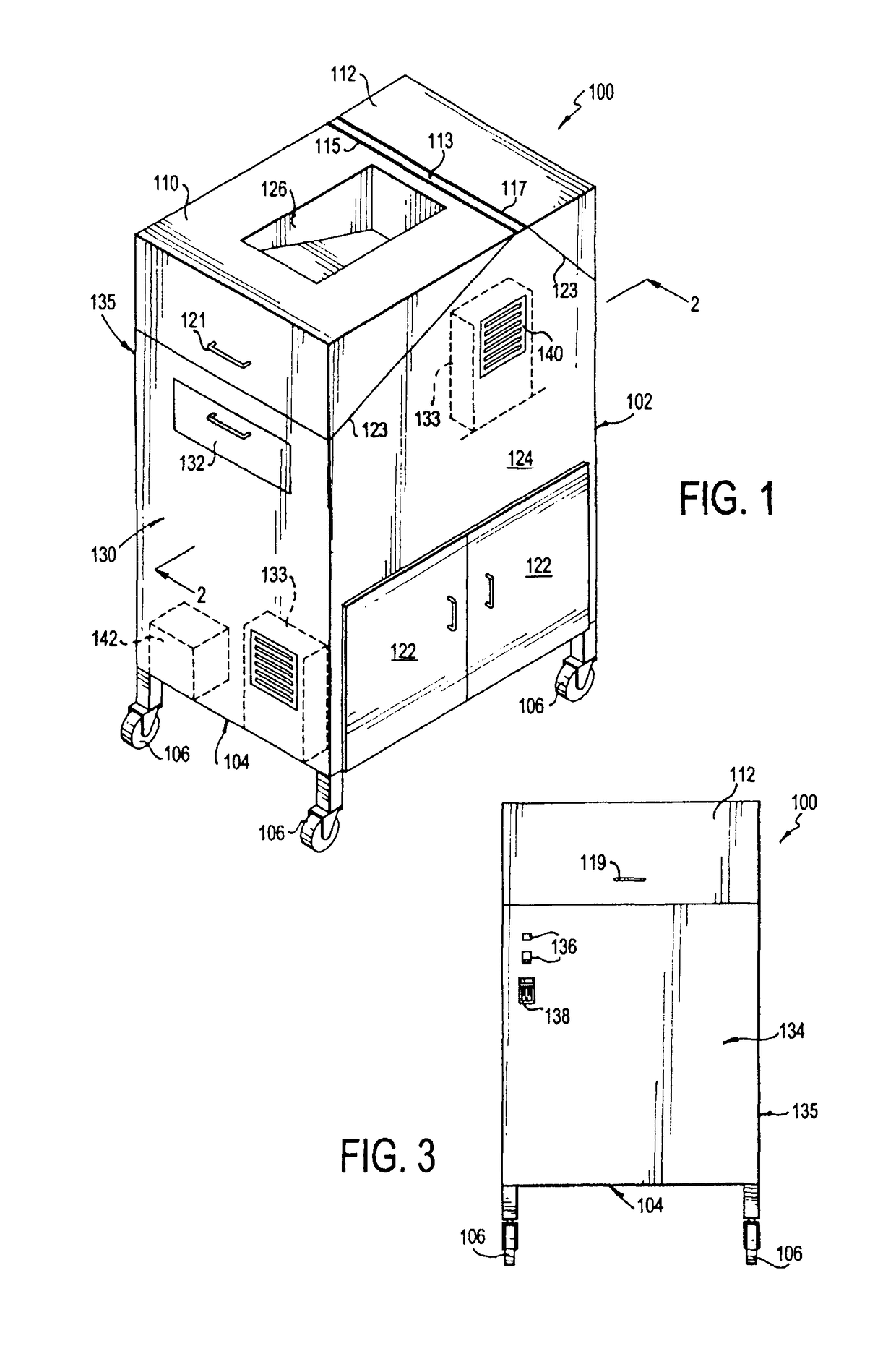
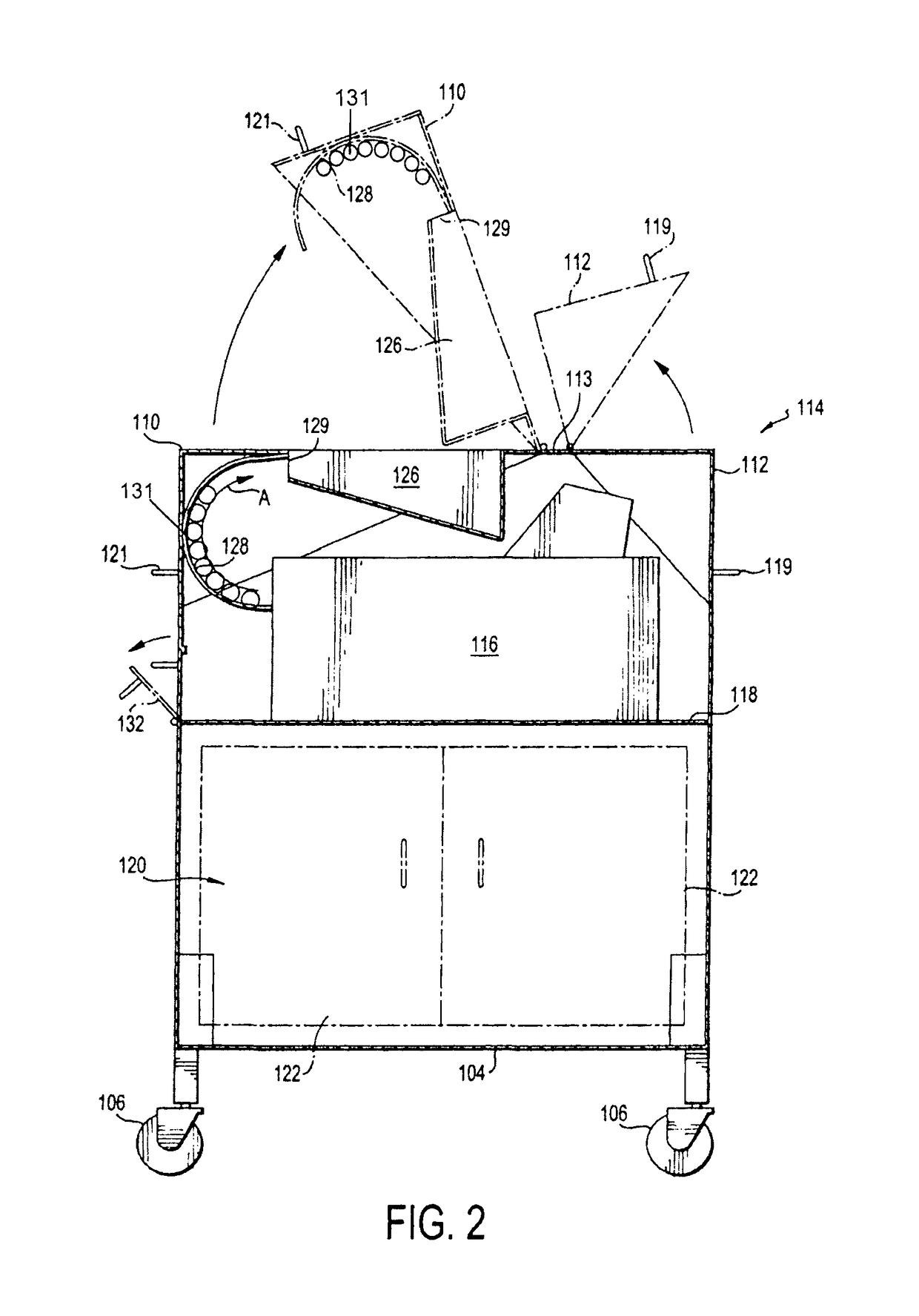
Portable cleanroom printing cabinet
Owner:VALTEK ASSOC INC
Cleanroom cleaning apparatus
InactiveUS20120066850A1Limiting and eliminating potential operator contaminationImprove accuracyTransportation and packagingNon-surface-active detergent compositionsEngineeringCleanroom
A cleanroom cleaning device includes a multi-compartment container having two or more compartments and one or more breakable seals with at least one compartment containing a cleaning implement and at least another compartment containing a liquid capable of saturating the cleaning implement when the seals are broken. Another cleanroom cleaning device includes a nonbreakable / nonburstable outer pouch, a breakable / burstable inner pouch containing a liquid, and a cleaning implement where the inner pouch containing liquid and the cleaning implement are both contained within the outer pouch.
Owner:FOAMTEC INT CO LTD
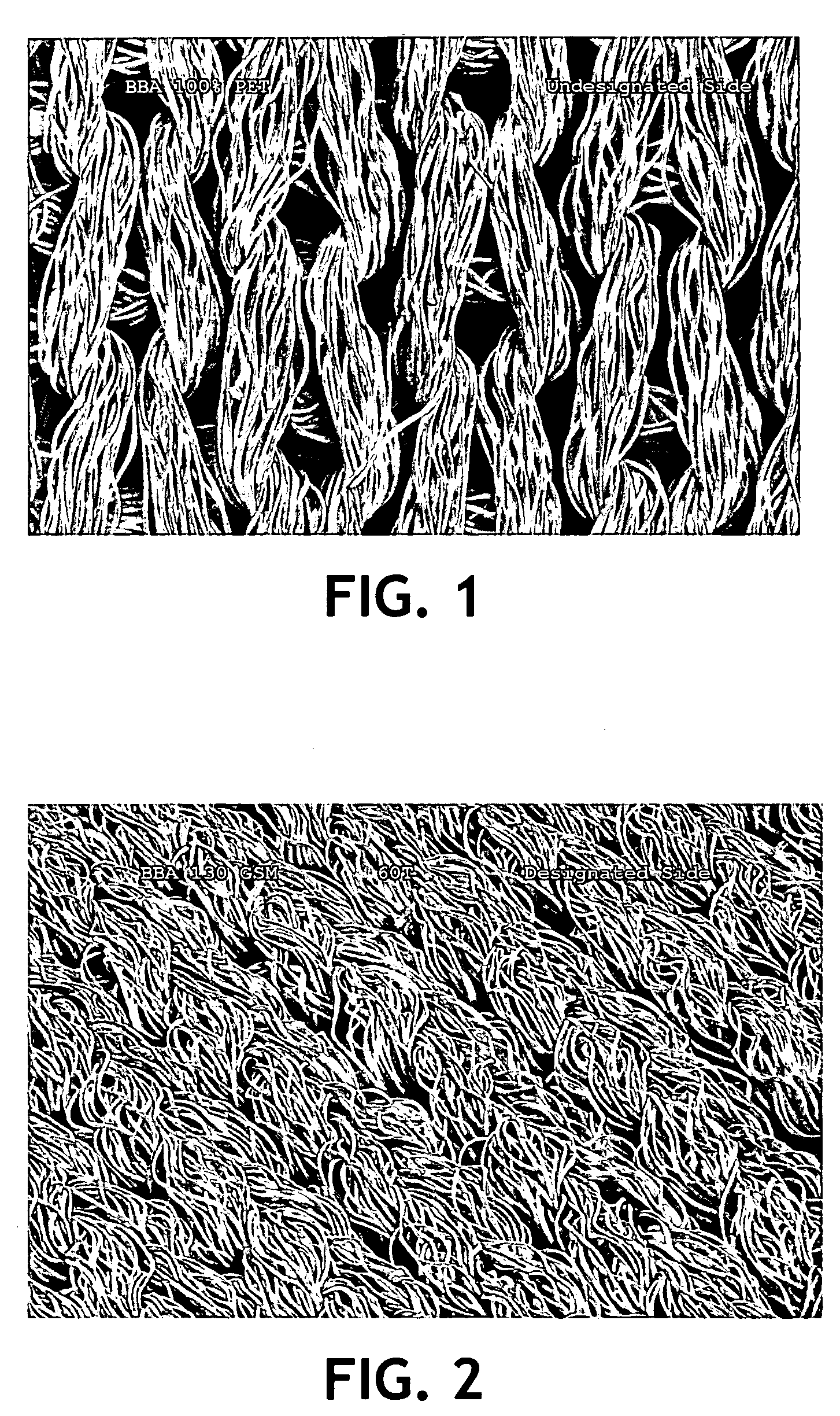
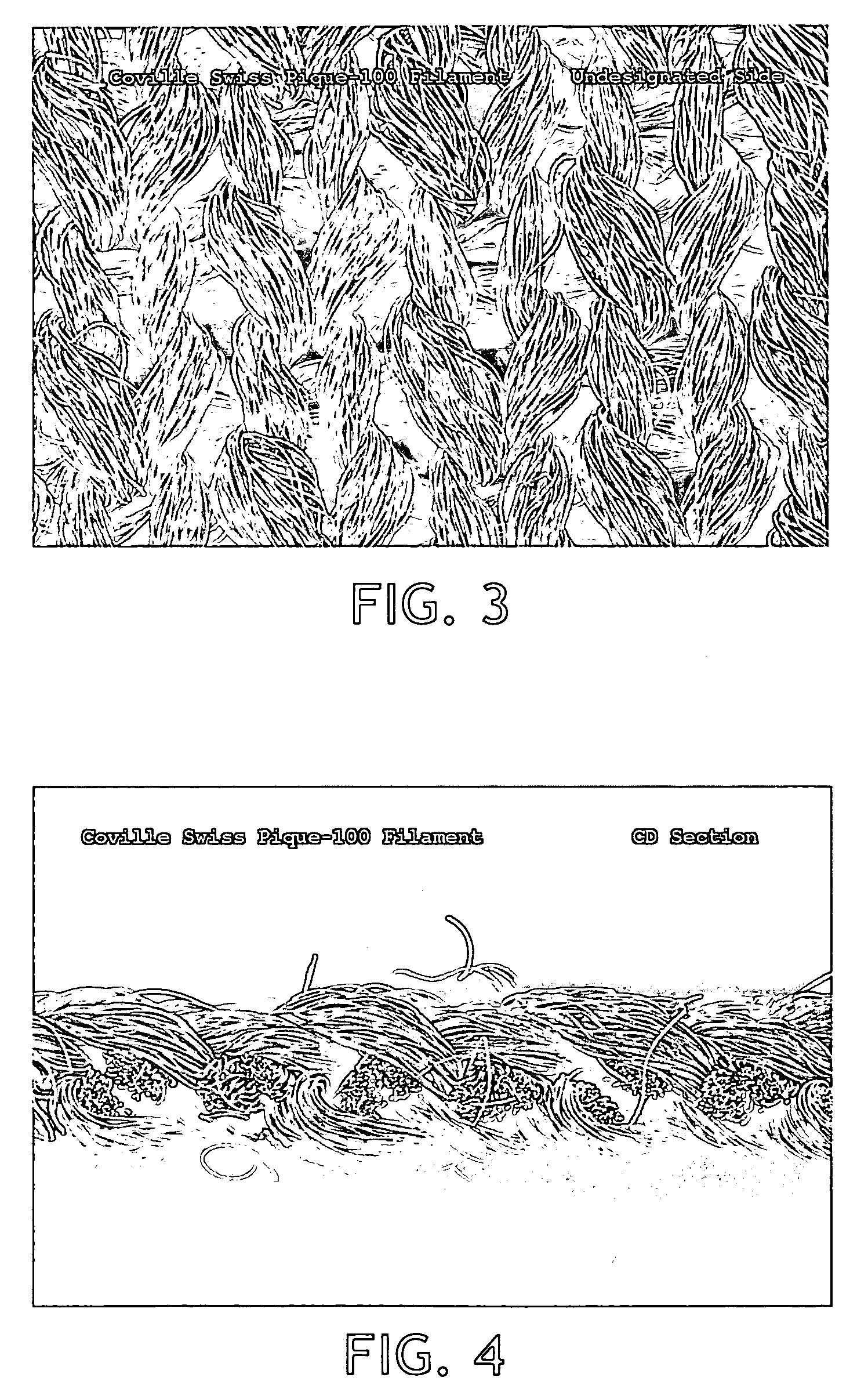
Cleanroom wiper
InactiveUS20100215905A1Quality improvementImprove cleanlinessLamination ancillary operationsCarpet cleanersFiberUltrasonic vibration
Owner:LIOU TIAN FA
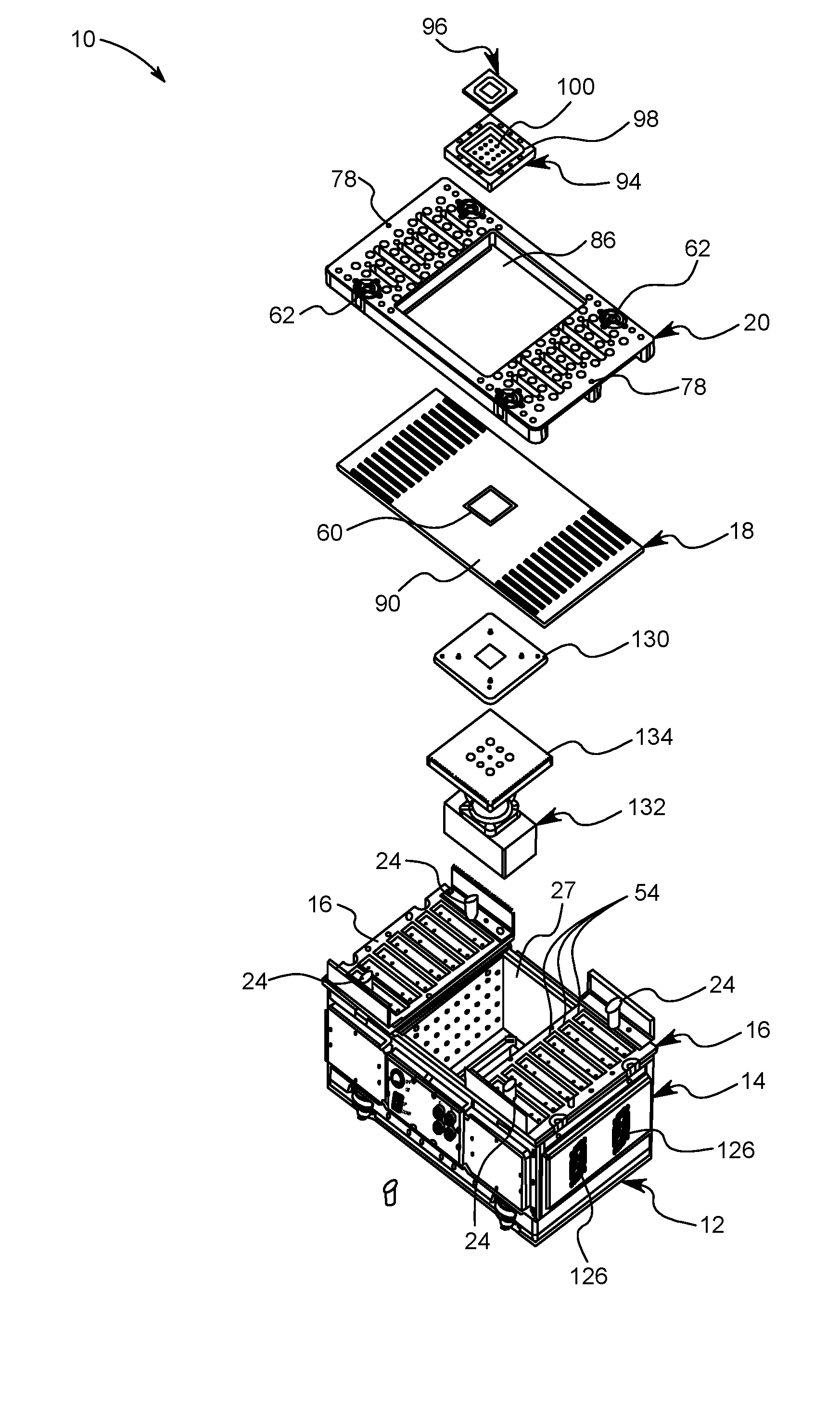
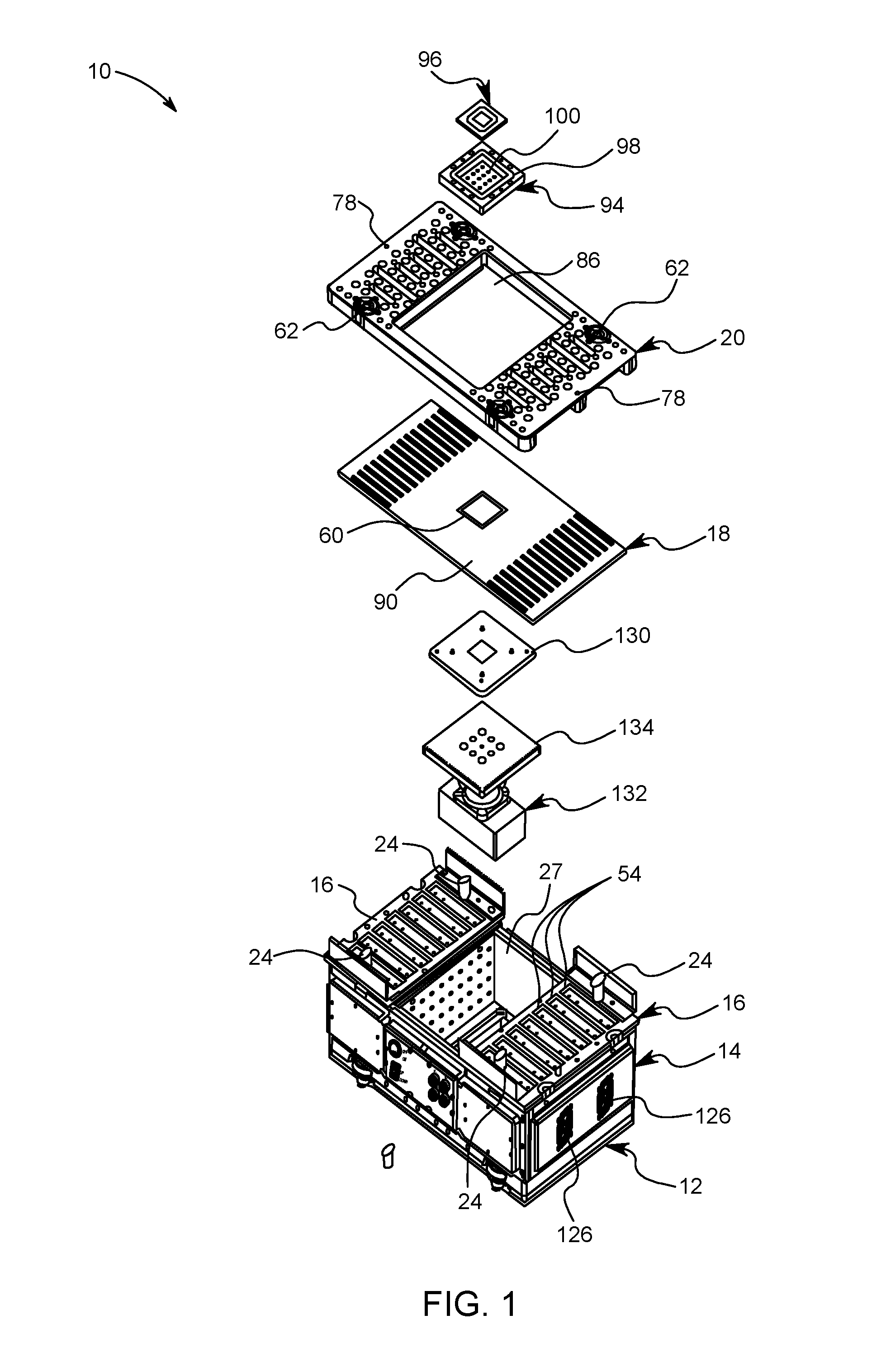
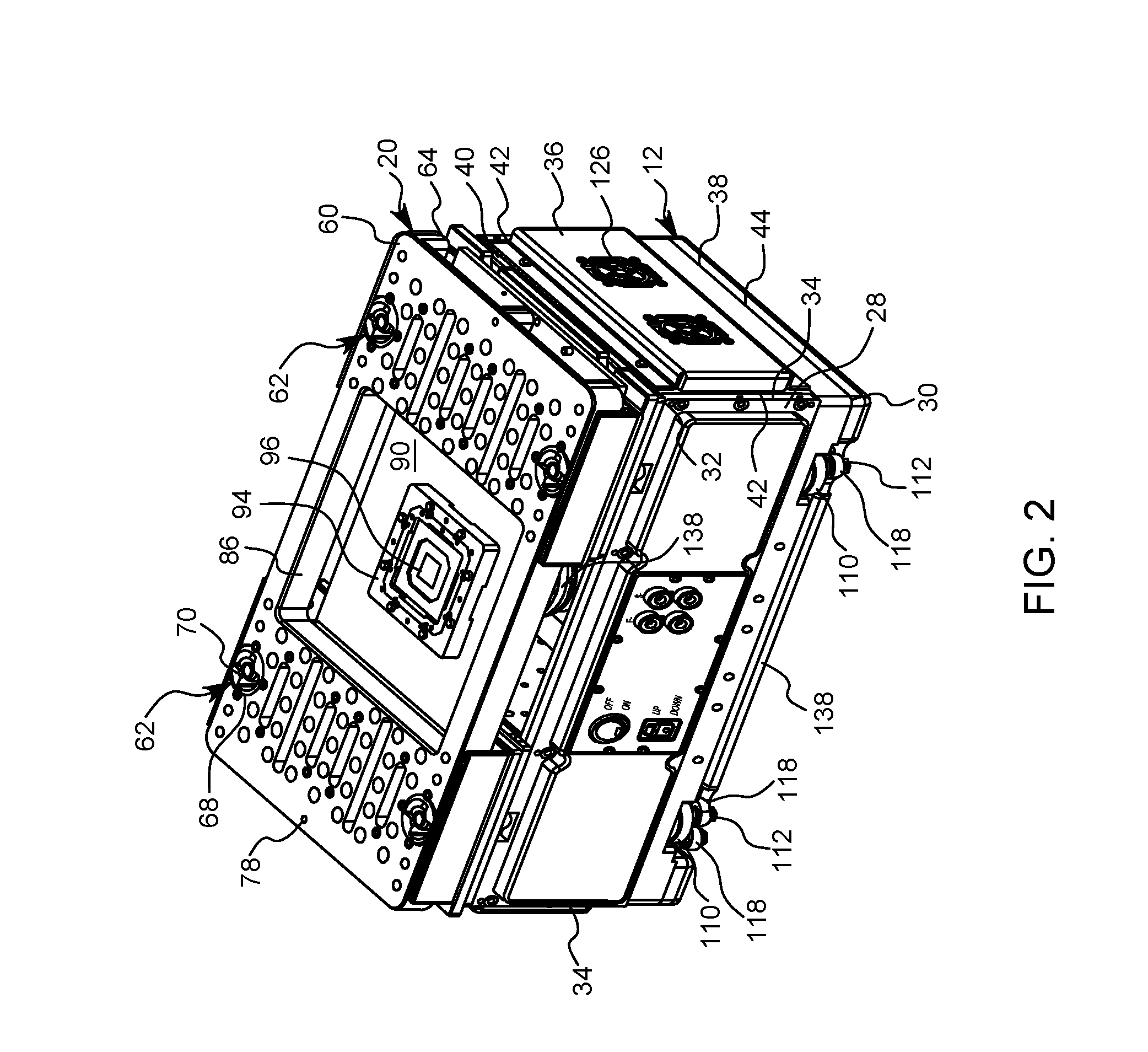
Quick change small footprint testing system and method of use
ActiveUS20160209443A1Quickly and easily removedQuickly and easily and replacedMeasurement instrument housingIndividual semiconductor device testingElectricityCapital equipment
A testing system for semiconductor chips having a removable device under test printed circuit board (DUT PCB) that electrically connects with the electrical testing components of the system. A removable top plate is placed on top of the DUT PCB and is locked in place by a plurality of locking posts that selectively connect to cam surfaces in the top plate that pull the top plate down sandwiching the DUT PCB between the top plate and the electrical testing components of the system. The DUT PCB is quickly and easily removed and replaced by moving the locking posts between an engaged position and a disengaged position. In this way, a single testing system can be used to test a great variety of semiconductor chips thereby reducing capital equipment costs and space needed in cleanrooms.
Owner:MODUS TEST
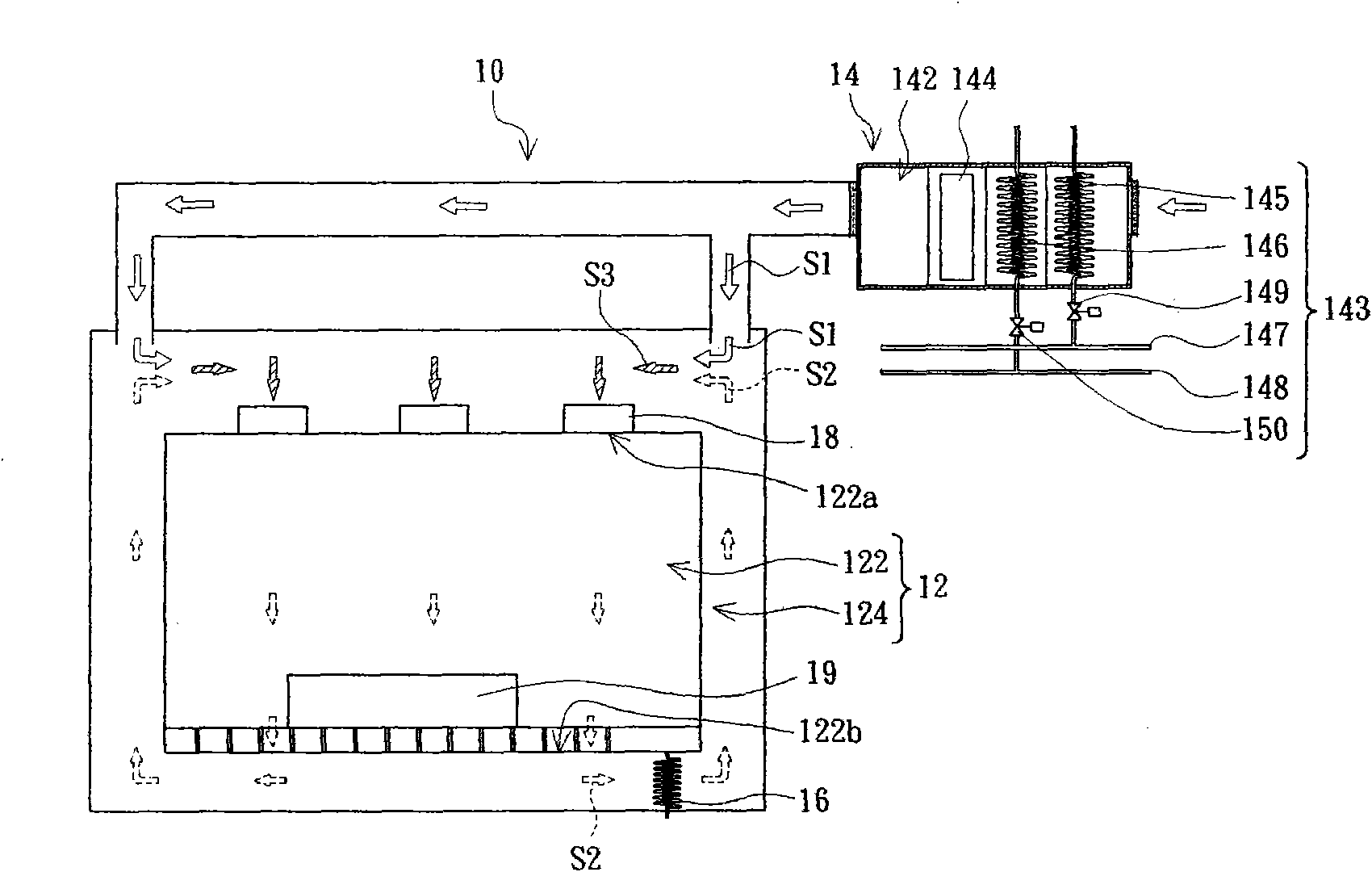
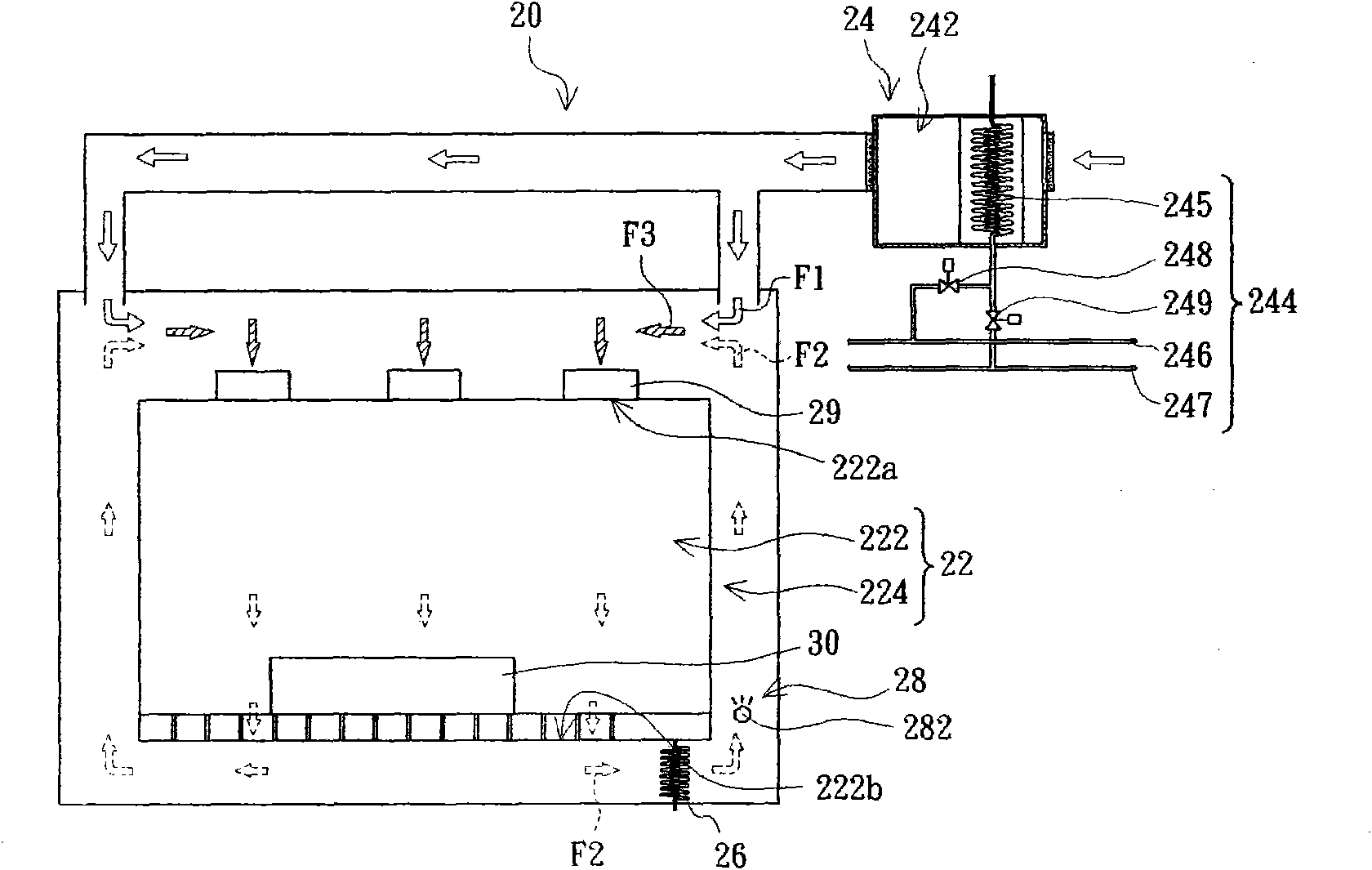
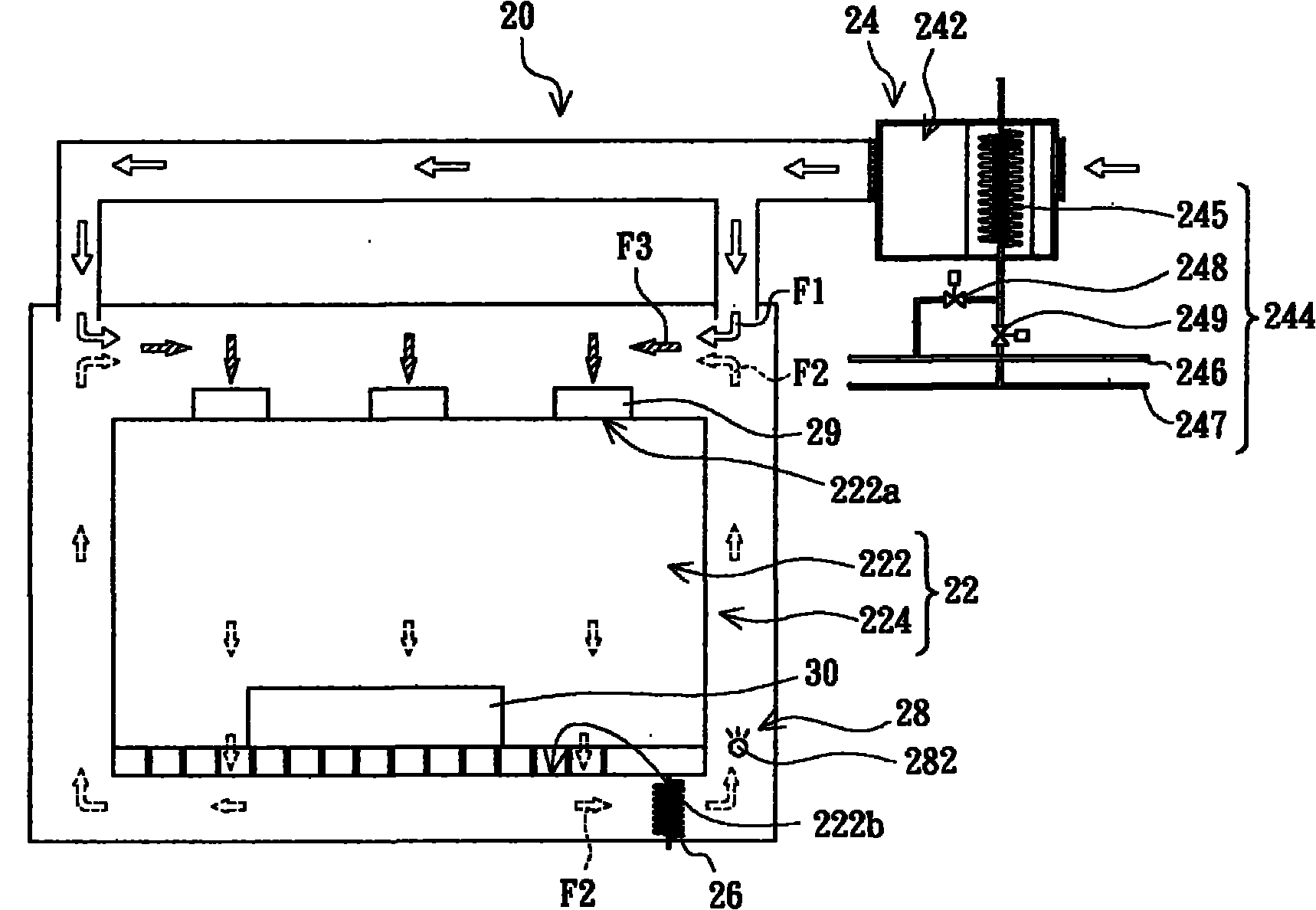
Cleanroom system and cleanroom air-conditioning method
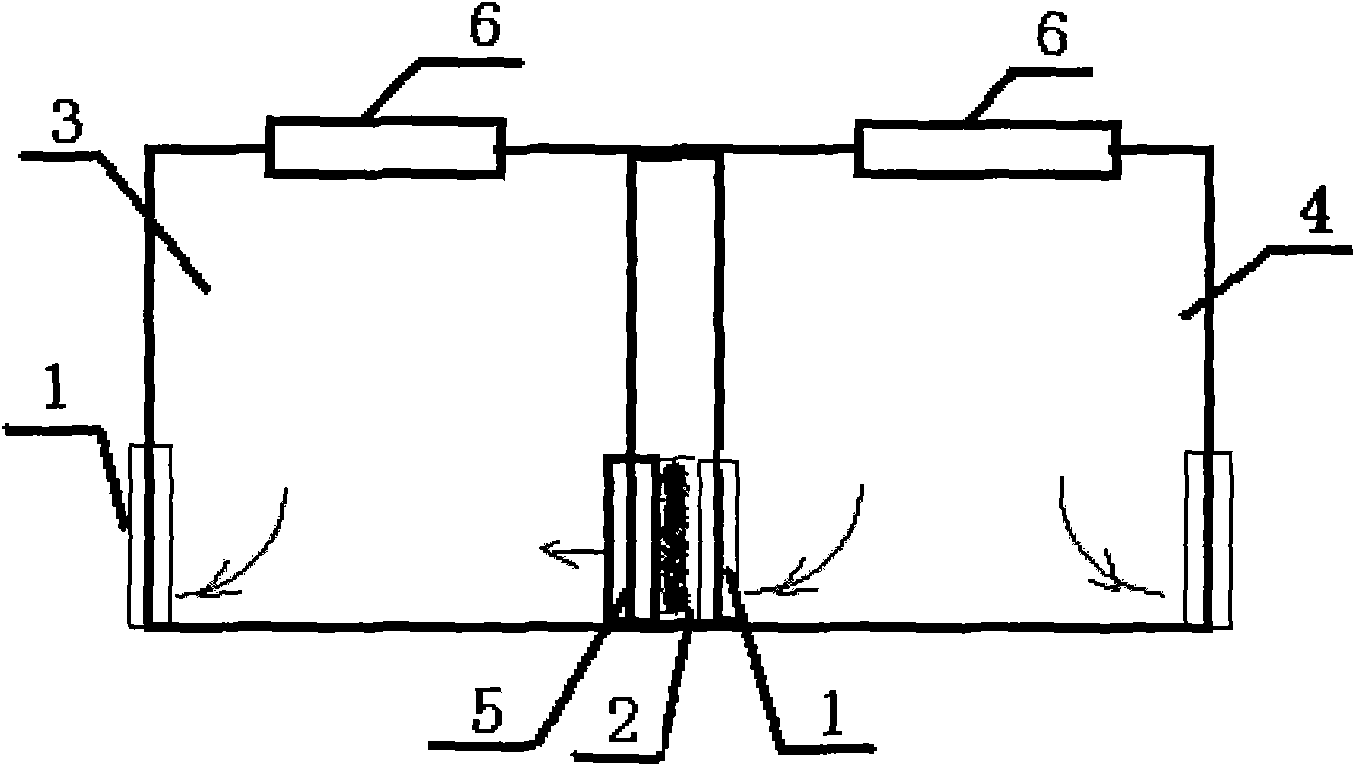
InactiveCN101922764AAchieve warmingAchieve coolingSpace heating and ventilation safety systemsLighting and heating apparatusThermostatHigh pressure water
The invention provides a cleanroom system comprising a cleanroom, a high-pressure water mist humidifier and an air conditioning device, wherein the cleanroom comprises a clean air chamber zone and an air return zone arranged on the periphery of the clean air chamber zone, the clean air chamber zone is provided with an air inlet and an air outlet, and the air return zone is communicated with the clean air chamber zone by the air inlet and the air outlet; and the high-pressure water mist humidifier is arranged in the air return zone. The air conditioning device comprises an air conditioning chamber and a thermostat, wherein the air conditioning chamber is communicated with the air return zone and is suitable to provide first air flow which flows through the inner part of the air conditioning chamber to enter the air return zone; the thermostat is used for regulating the temperature of the first air flow; second air flow enters the air return zone after leaving the clean air chamber zone from the air outlet, is humidified and cooled by the high-pressure water mist humidifier in the air return zone and is combined with the first air flow to form third air flow; and the third air flow enters the clean air chamber zone from the air inlet. In addition, the invention also provides a cleanroom air-conditioning method.
Owner:AU OPTRONICS CORP
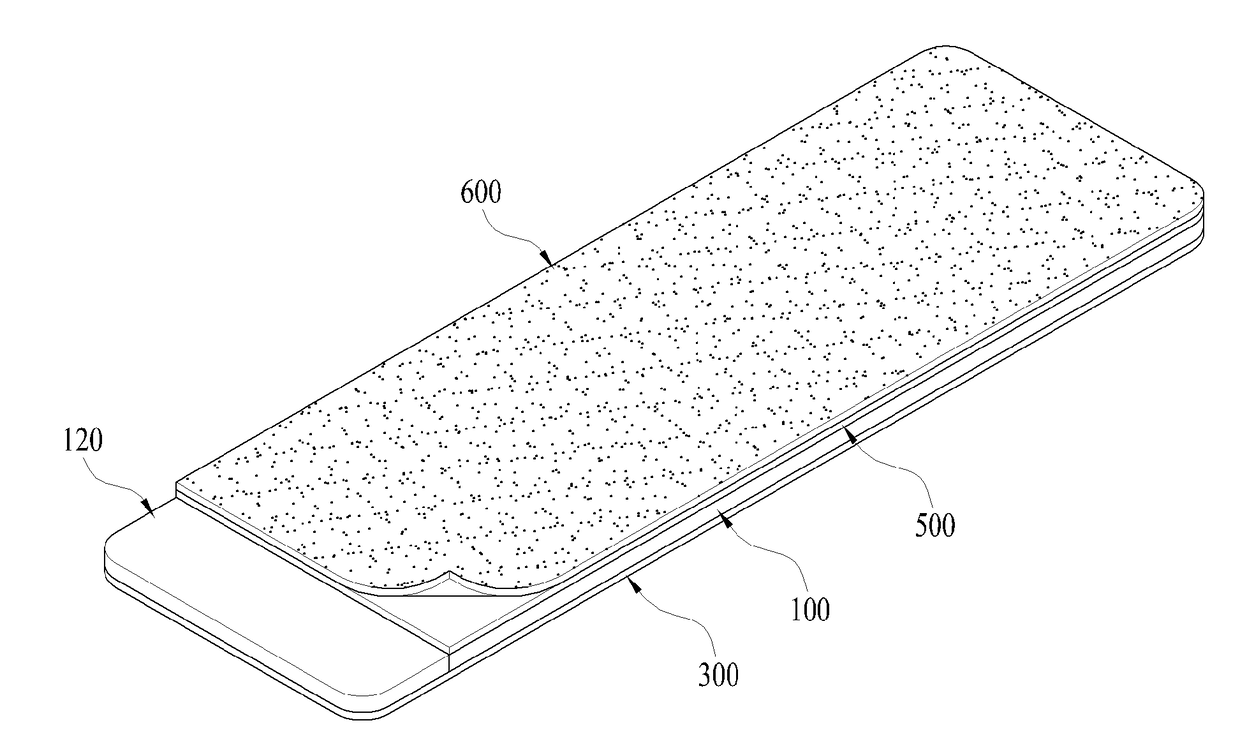
Test film for detecting surface particles in clean room
ActiveUS20170315038A1Easy to confirmEasy to cleanWithdrawing sample devicesOptically investigating flaws/contaminationAdhesiveEngineering
The present invention relates to a test film for detecting surface particles in a clean room in order to prevent inferior products by measuring the contamination level of the clean room using the surface particles. The present invention provides a test film for detecting surface particles in a cleanroom, the test film comprising a substrate which has a predetermined thickness and is formed of a transparent synthetic resin material; a first adhesive layer which is formed at one side of the substrate and collects the surface particles; a release film which is adhered to the first adhesive layer and is separated from the first adhesive layer when the surface particles are collected; a second adhesive layer which is formed at the other side of the substrate; and a protective film which is adhered to the second adhesive layer so as to protect the substrate and has gradations indicated thereon. According to the present invention, there is an effect in that it is possible to easily and quickly check whether or not a clean room is contaminated by collecting, on the adhesive layer-applied surface of a substrate to which an adhesive is applied, surface particles adhered to an object to be measured, and then measuring the number, size, distribution, etc. of the surface particles with the naked eye or using instruments such as a light, a magnifier, a microscope, or the like.
Owner:JEDEX
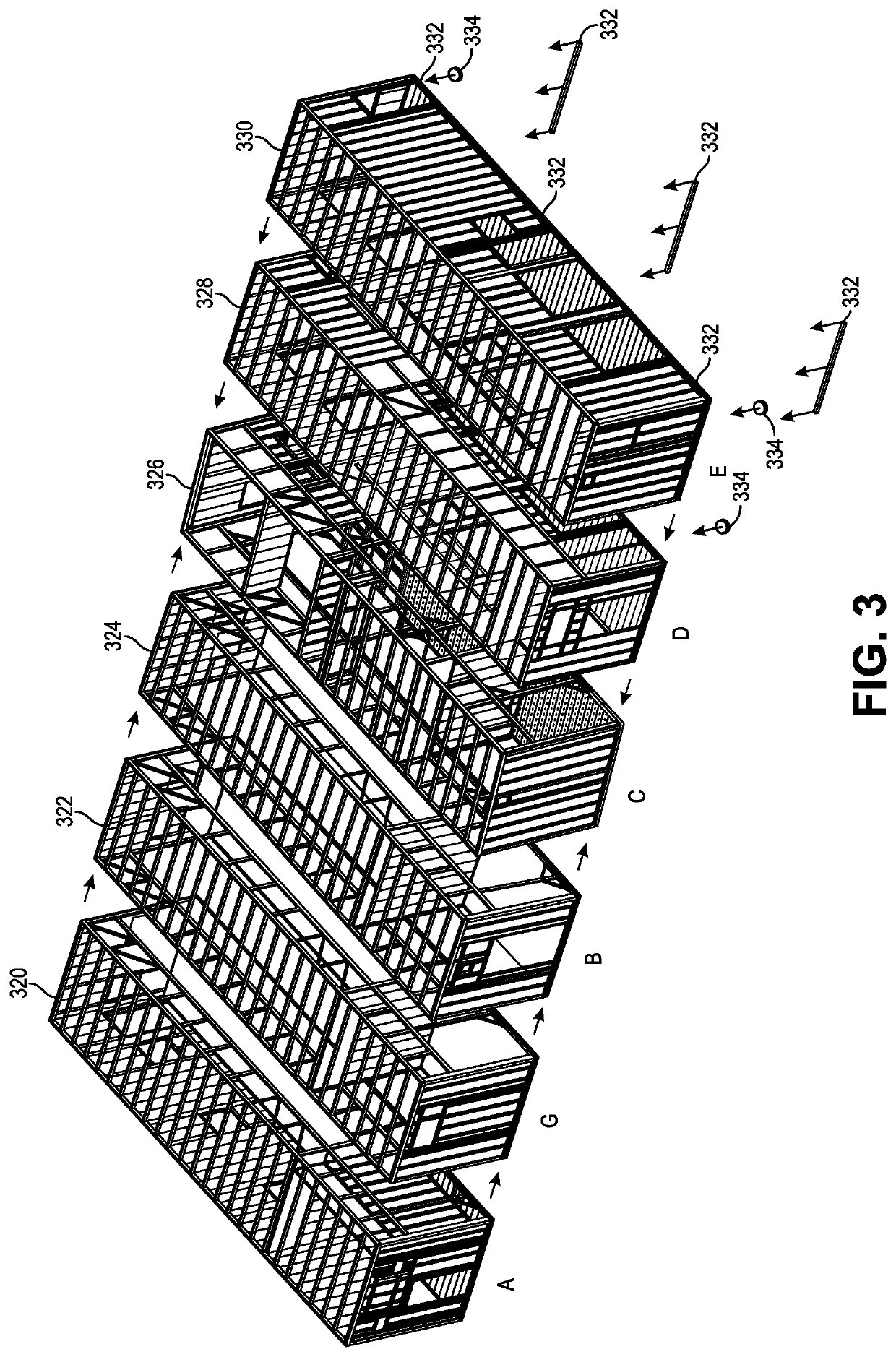
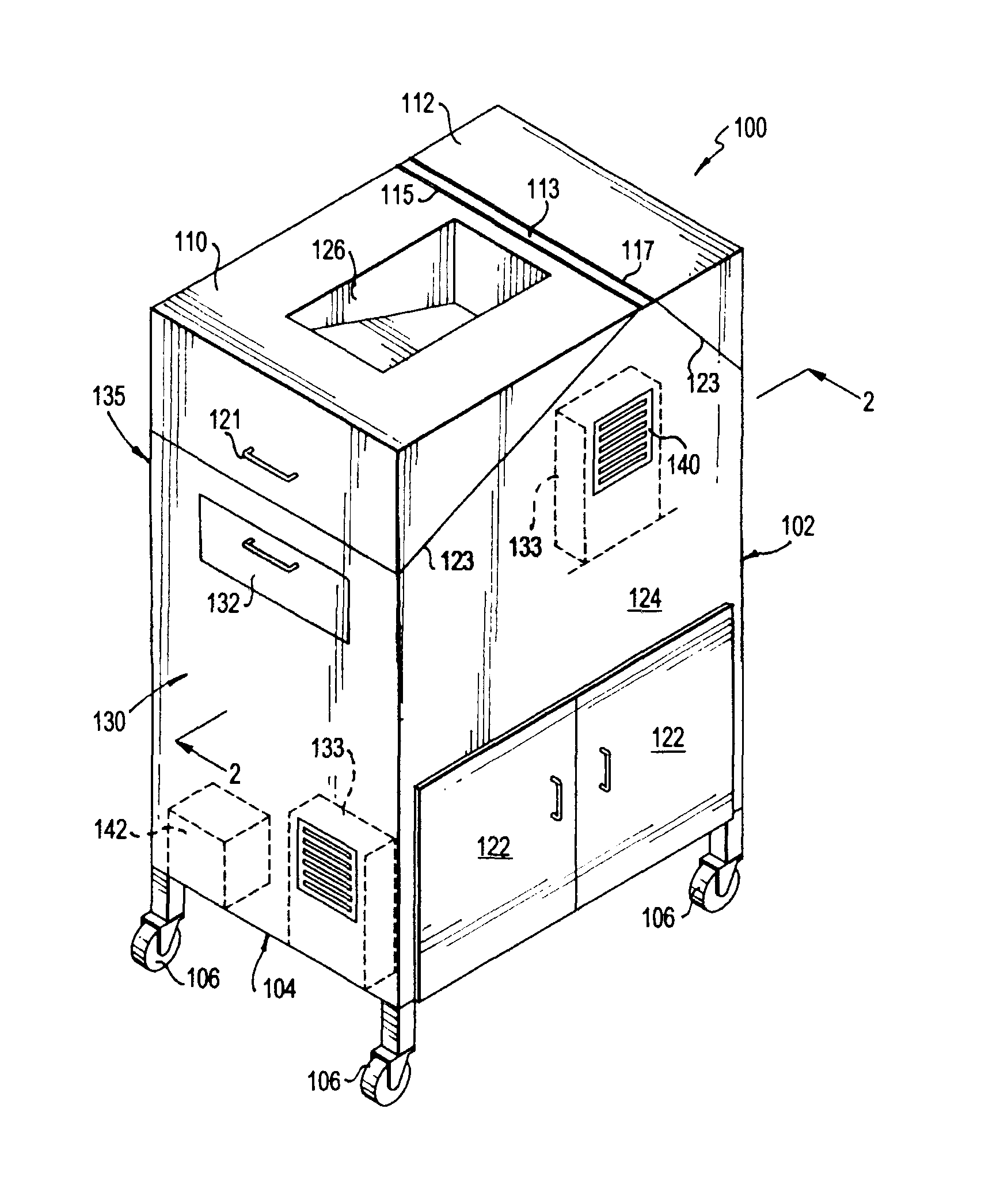
Ballroom-Style Cleanroom Assembled from Modular Buildings
ActiveUS20220064935A1Easy to joinMechanical apparatusDust-free enclosuresArchitectural engineeringJackscrew
A rapidly deployable cleanroom is constructed from multiple modular building sections that are side-by-side and have similarly sized lengths, as well as user-accessible panels in multiple of them for air, water, power, networking, and other utilities at a similar internal location along their lengths. The utility panels can be standardized such that each panel is substantially identical to one another. The modular building sections can be drawn together using tension cables within lateral hollow extruded structural tubes beneath and / or above the cleanroom, the tension system utilizing jackscrews to pull everything tight. Because the utility panels are at the same longitudinal location in the building sections, the joined structure and resulting open space “ballroom” style cleanroom has the utility panels aligned with each other inside the cleanroom.
Owner:G CON MFG
Cleanroom wiper
A wiper for use in a cleanroom environment made of a knitted, continuous synthetic filaments is disclosed. The wiper has a surfactant added to the surface of the knitted substrate. The wiper has improved wiping ability, low lint and low extractable ions making it suitable for use in critical cleanroom environments.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Overalls for cleanroom and the like
The invention relates to overalls (1) for a cleanroom including at least one grasping means (16) on an inner surface to facilitate dressing.
Owner:倍微米公司
Filter housing
ActiveUS20170276400A1Achieving a sealed connectionFacilitates filter replacementMechanical apparatusGas treatmentAir filterFilter media
The present invention relates to an air filter housing for supplying air to a space, said air filter housing being configured to be arranged in a ceiling of said space, said housing having a mouth facing downward, said mouth comprising a sealing surface configured to receive a panel type air filter comprising a frame and a filter medium held within said frame, such that a sealing connection between said sealing surface and said frame is achieved, characterized in that said filter housing further comprises at least one resilient member arranged below the sealing surface such that when a panel type air filter is inserted into the mouth, the resilient member will be deformed by the filter frame as the filter passes the resilient member, and resume its original shape once the filter has passed, such that the filter is retained between the sealing surface and the resilient member. The present invention further relates to the use of a filter housing in a cleanroom, and to a method of mounting a panel type air filter in an air filter housing.
Owner:CAMFIL AB
Cleanroom workstation particle capture system
Owner:SWAN JAWN P +2
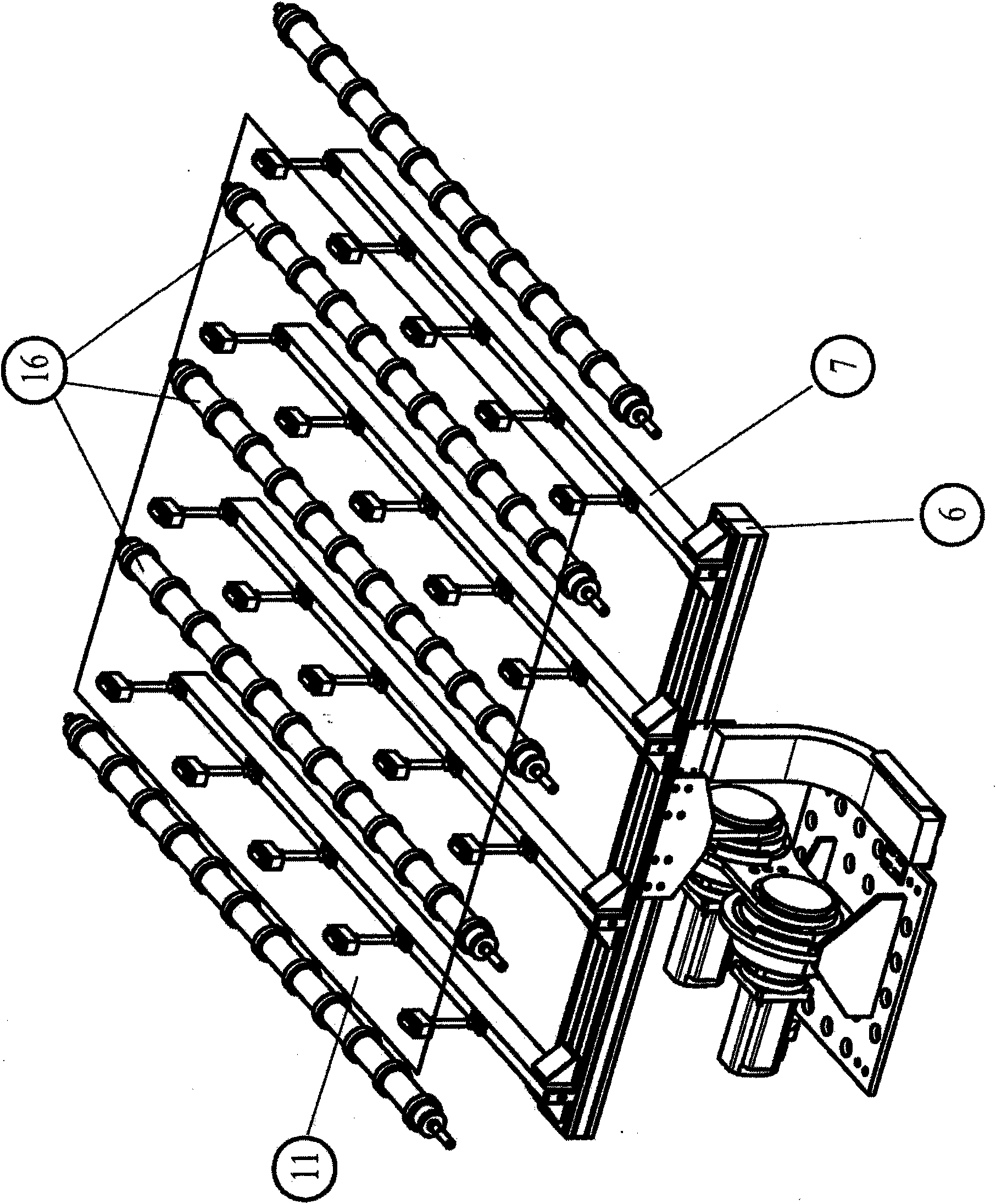
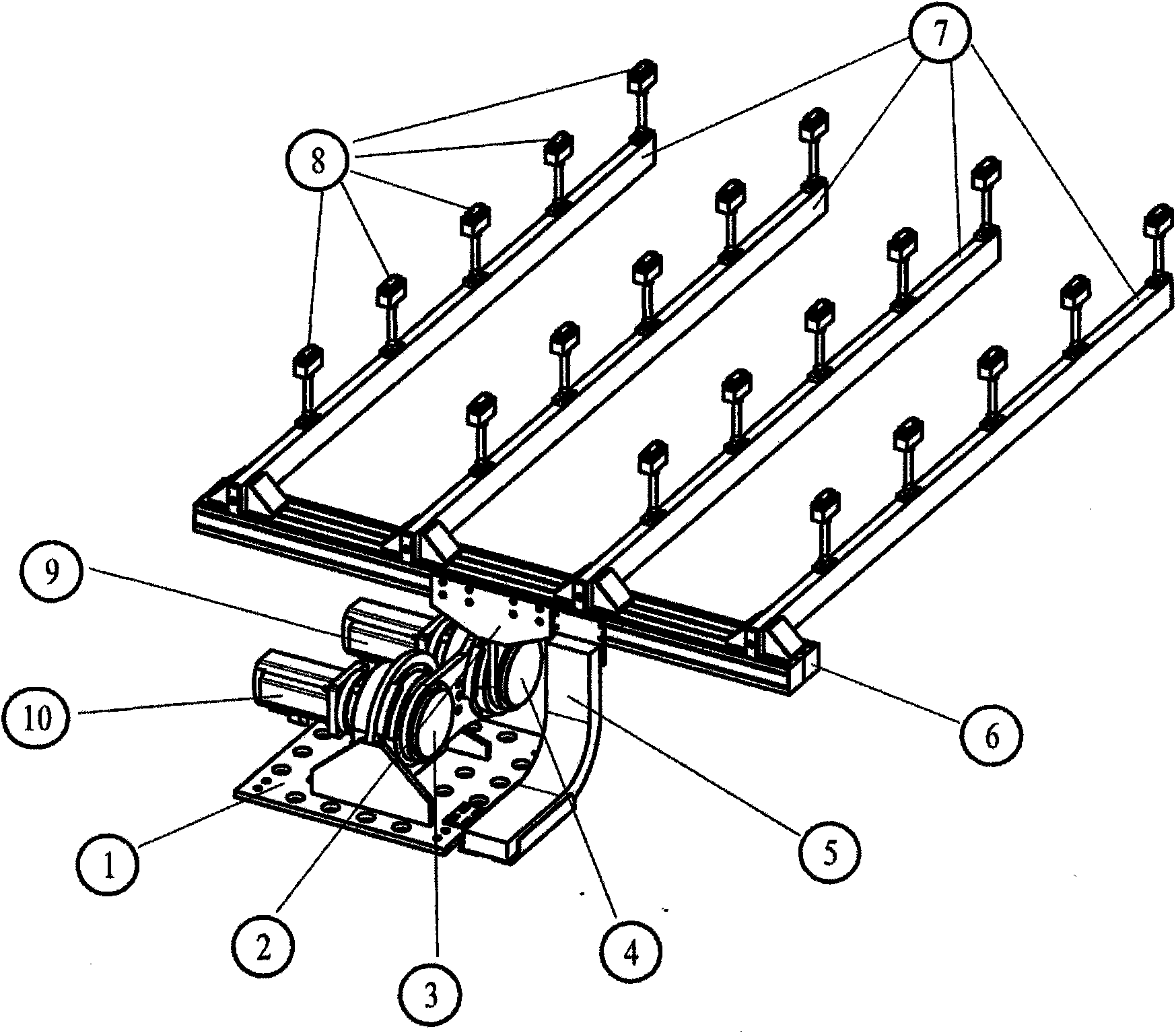
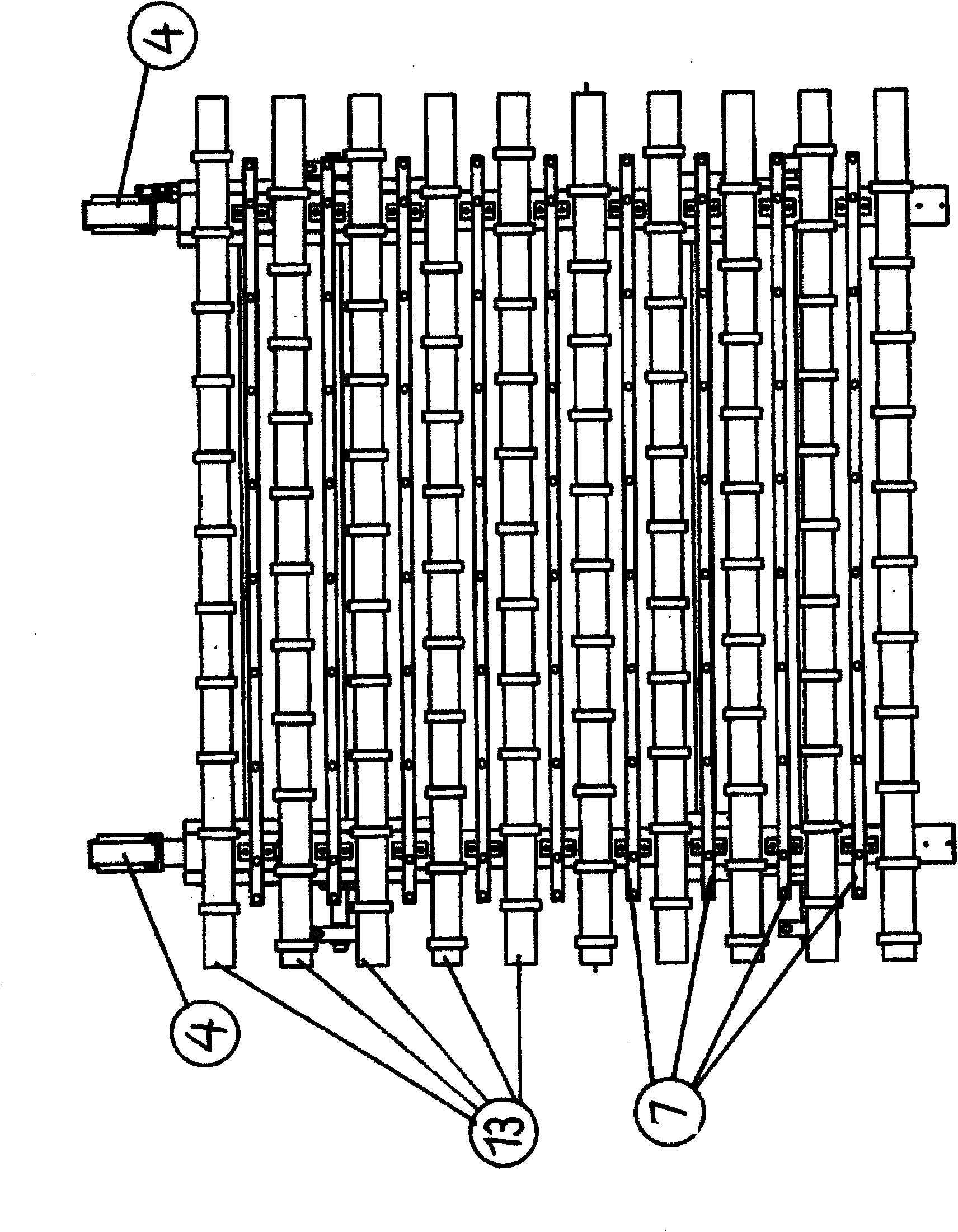
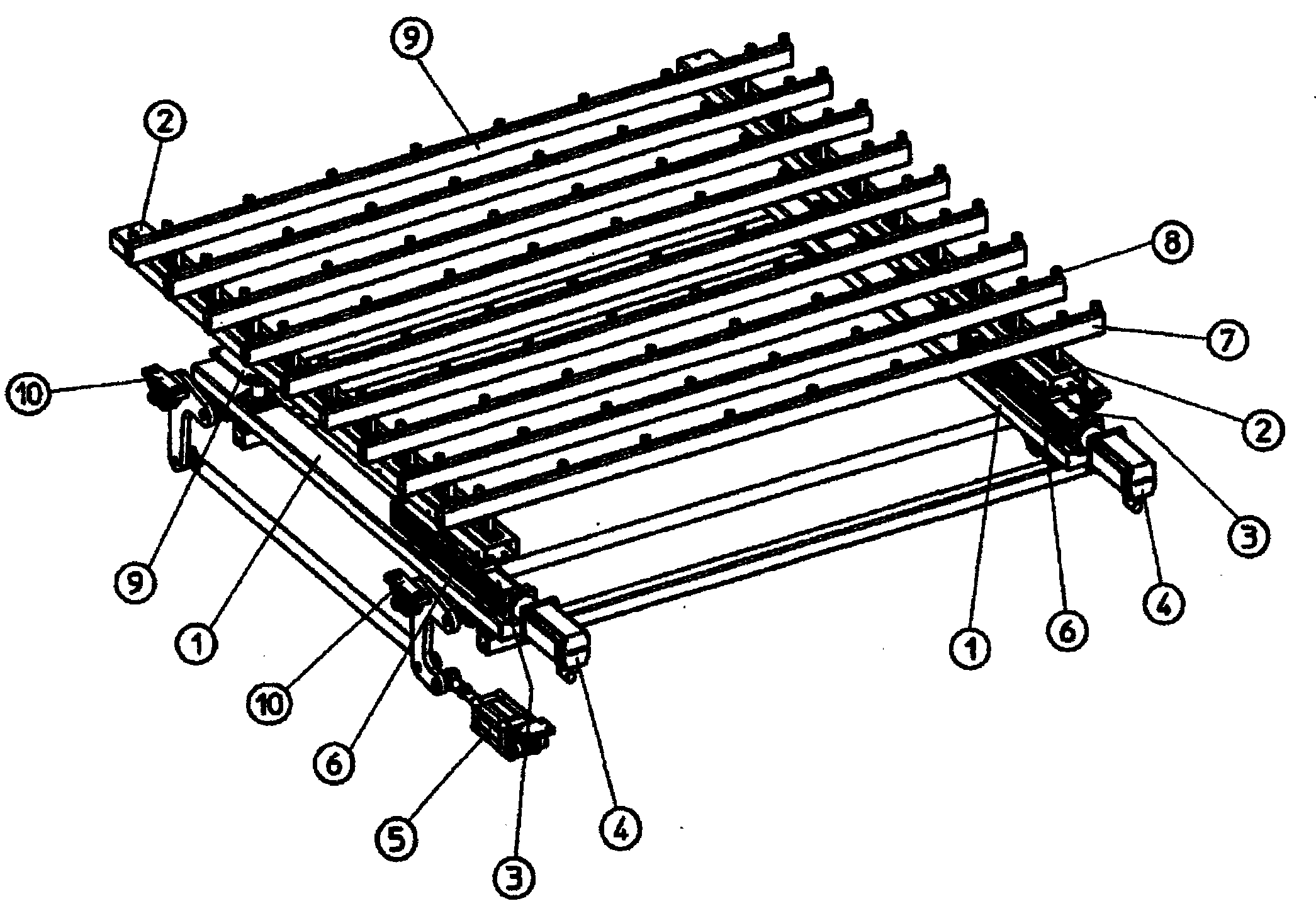
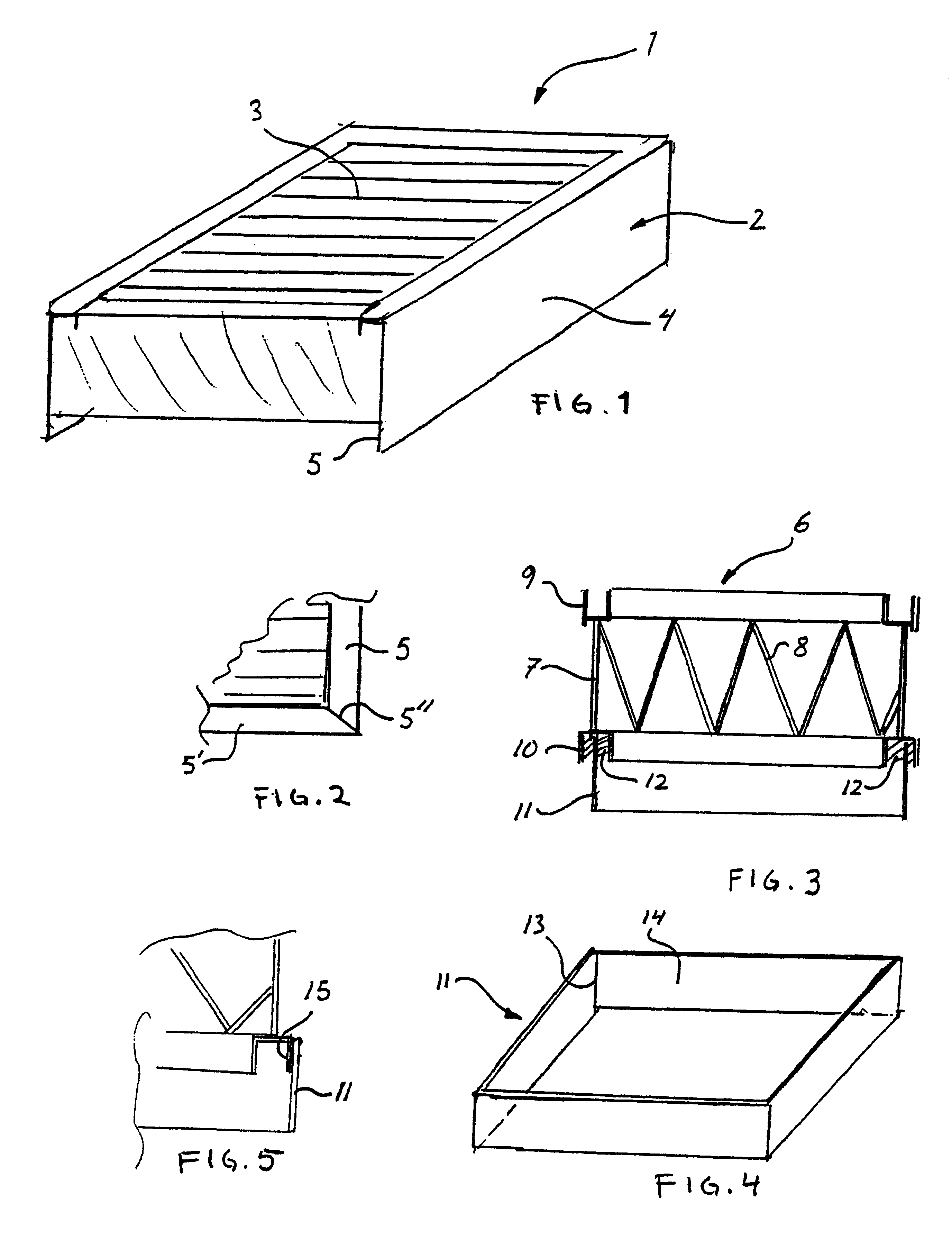
Apparatus and method for transferring shock-sensitive glass plates in ultra clean rooms
Disclosed are a method and an apparatus for the contamination-free transfer of thin, shock-sensitive crystalline plates, especially glass plates (11), from a horizontal into a defined vertical position. Said method and apparatus have the following features: a) a transverse bar (6) of a transfer fork is disposed below the glass plate (11) in order to grip the glass plate (11), said transverse bar (6) being fitted with suction head-supporting bars (7) which are vertically mounted thereon and have suction heads (8) that are distributed along the top side thereof and penetrate the free space between the rolls; b) the suction heads (8) are moved close to the bottom side of the glass plate (11), and the suction elements (15) thereof are connected to the glass plate (11) by means of intake air; c) the glass plate (11) is put into the placement device (12) after being finely adjusted. Also disclosed are a computer program and a machine-readable carrier containing the corresponding program code.
Owner:GRENZEBACH MASCHINENBAU GMBH
Illuminating filter for particle controlled environments
InactiveUS20120180667A1Simple lightingError minimizedCombination devicesMechanical apparatusLow voltageFiltration
An illuminating filter which provides both clean air and light to a particle controlled environment. The integration of filtration and lighting simplifies the design of cleanrooms, mini-environments, and clean zones. Space savings and cost savings are served by combining filtration and lighting into a single structure. Light emitters, such as LEDs or other solid state devices, may be used where low voltage or low current is desirable.
Owner:OTTESEN LARRY M +1
Ventilation system for clean rooms
InactiveCN101644470AHigh power consumptionReduce pollutionLighting and heating apparatusSpace heating and ventilation detailsAir volumeEngineering
The invention provides a ventilation system for clean rooms, which comprises wind inlet units, wind outlet units and a control unit, wherein a wind replenishing opening is arranged below a clean roomwith a lower clean level; the wind replenishing opening is connected with the wind inlet unit of the clean room with a lower clean level and is connected with the wind outlet unit of an adjacent cleanroom with a higher clean level; and filter screens are arranged in the middle of the ventilation system, and the apertures of the filter screens based on different clean levels are different. The wind inlet unit of the clean room with a lower clean level regulates the wind inlet quantity according to the wind replenishing quantity without influencing the wind outlet quantity of the wind outlet unit, and by analogy, the wind exhaustion of a clean room with a highest clean level is used as a primary wind replenishing source, the wind exhaustion is thoroughly finished until a wind outlet unit with a lowest clean level is replenished. The invention has the effects that the wind replenishing opening is arranged below the clean room, thus large-area turbulent flow of the upward air can not occur, the wind outlet quantity is reduced simultaneously, and a larger amount of electric quantity is saved.
Owner:WUJIANG HONGDA VENTILATION REFRIGERATION EQUIP FACTORY
Portable cleanroom printing cabinet
ActiveUS9566811B2Other printing apparatusServing tablesElectrical and Electronics engineeringCleanroom
Owner:VALTEK ASSOC INC
Method and device for decreasing contamination
InactiveCN101137325AReduce surrounding pollutionParticle size analysisDiagnostic recording/measuringMicroparticleViscoelasticity
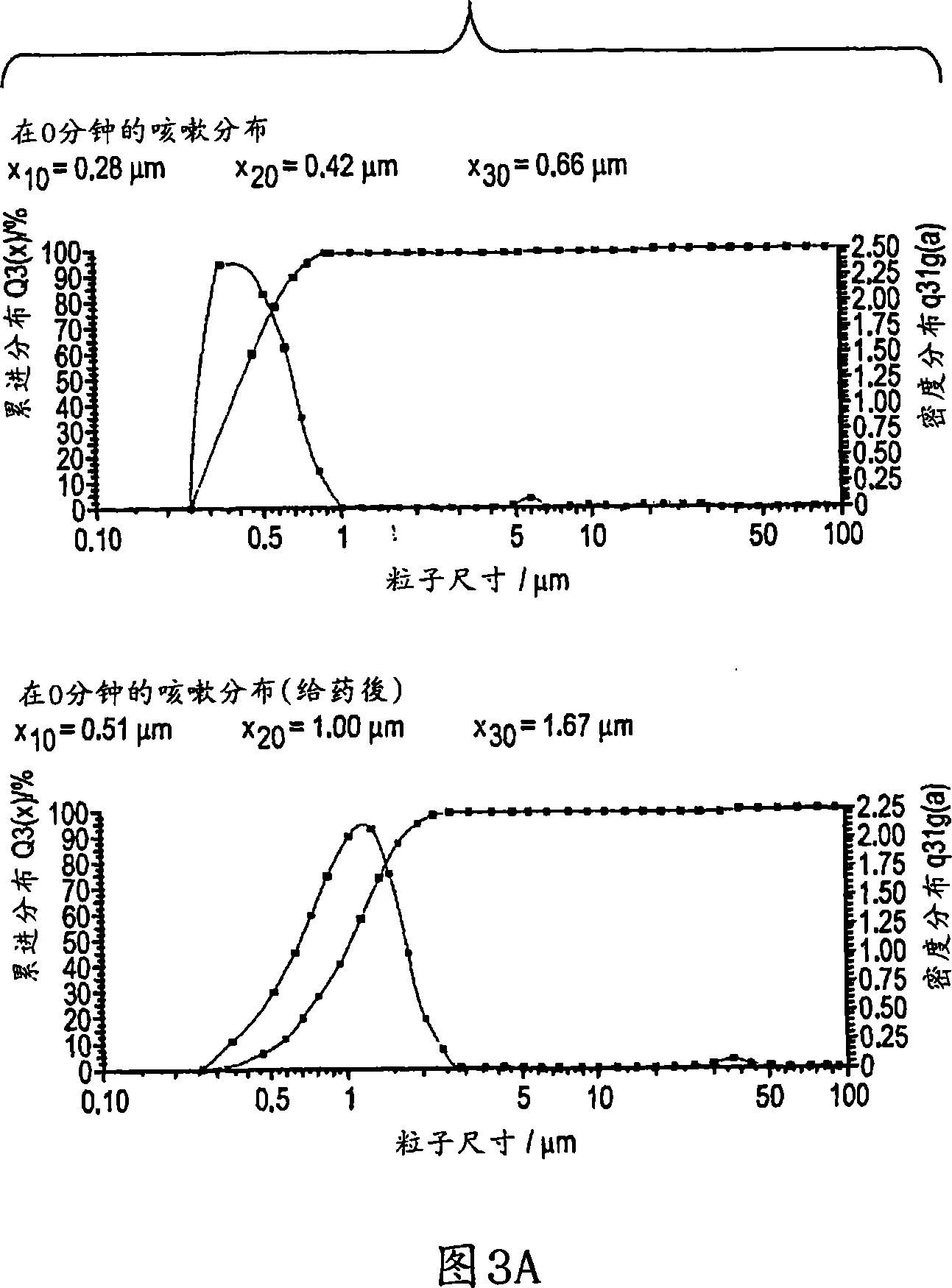
Methods and devices to determine rate of particle production and the size range for the particles produced for an individual are described herein. The device ( 10 ) contains a mouthpiece ( 12 ), a filter ( 14 ), a low resistance one-way valve ( 16 ), a particle counter ( 20 ) and a computer ( 30 ). Optionally, the device also contains a gas flow meter ( 22 ). The data obtained using the device can be used to determine if a formulation for reducing particle exhalation should be administered to an individual. This device is particularly useful prior to and / or following entry in a cleanroom to ensure that the cleanroom standards are maintained. The device can also be used to identify animals and humans who have an enhanced propensity to exhale aerosols (referred to herein as ''over producers'', ''super-producers'', or ''superspreaders''). Formulations to reduce particle production are also described herein. The formulation is administered in an amount sufficient to alter biophysical properties in the mucosal linings of the body. When applied to mucosal lining fluids, the formulation alters the physical properties such as the gel characteristics at the air / liquid interface, surface elasticity, surface viscosity, surface tension and bulk viscoelasticity of the mucosal lining. The formulation is administered in an effective amount to minimize ambient contamination due to particle formation during breathing, coughing, sneezing, or talking, which is particularly important in cleanroom applications. In one embodiment, the formulation for administration is a non-surfactant solution. In one embodiment, the formulations are conductive formulations containing conductive agents, such as salts, ionic surfactants, or other substances that are in an ionized state or easily ionized in an aqueous or organic solvent environment. Preferably the formulation is administered in the form of an aerosol.
Owner:PULMATRIX
Portable cleanroom printing cabinet
Owner:VALTEK ASSOC INC
Selective wet etching of gold-tin based solder
ActiveUS20090050903A1Simple methodNot contaminate equipmentSolid-state devicesSemiconductor/solid-state device manufacturingEtchingCompound (substance)
The present invention is directed to post-deposition, wet etch processes for patterning AuSn solder material and devices fabricated using such processes. The processes can be applied to uniform AuSn layers to generate submicron patterning of thin AuSn layers having a wide variety of features. The use of multiple etching steps that alternate between different mixes of chemicals enables the etch to proceed effectively, and the same or similar processes can be used to etch under bump metallization. The processes are simple, cost-effective, do not contaminate equipment or tools, and are compatible with standard cleanroom fabrication processes.
Owner:CREE INC
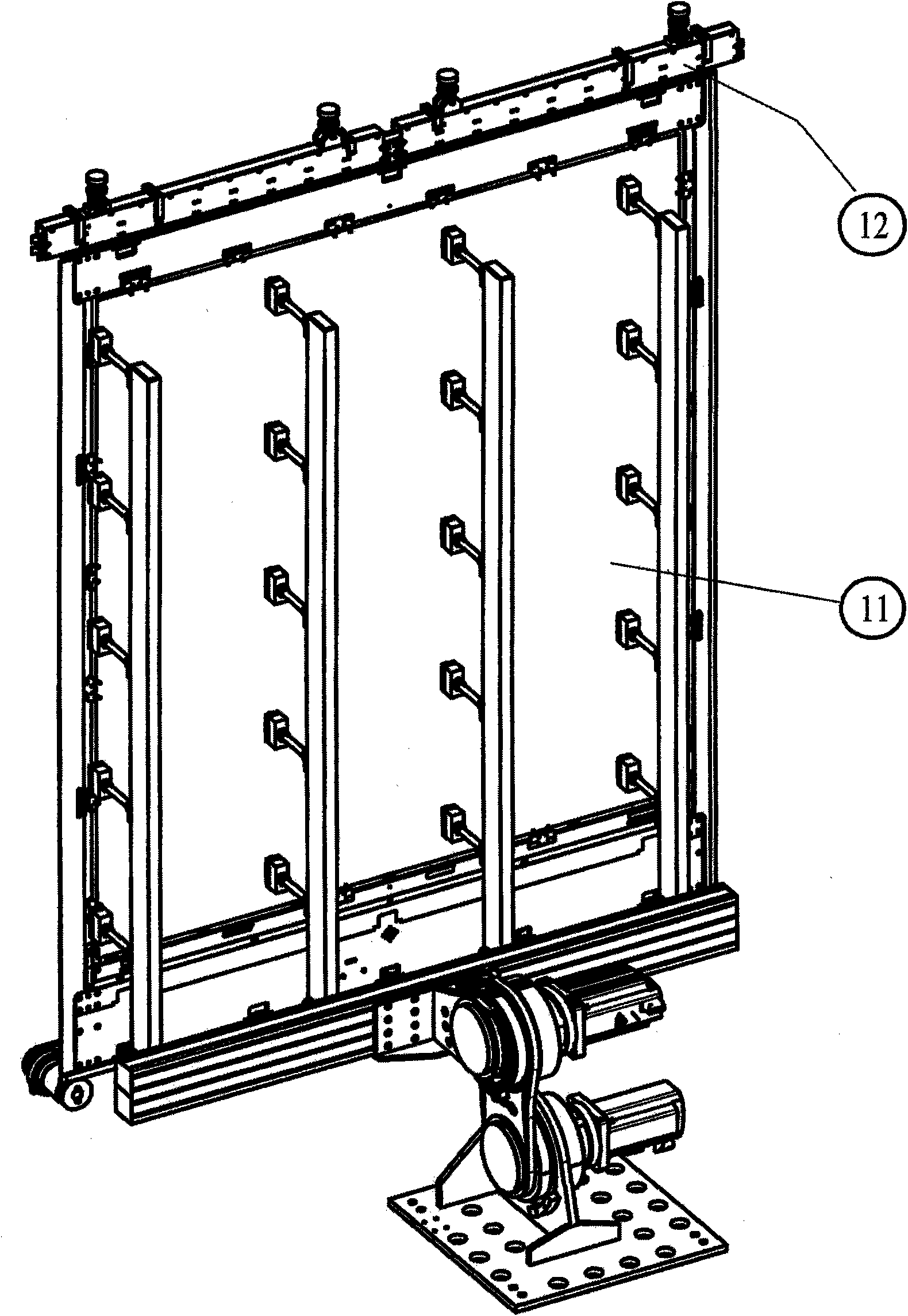
Apparatus and method for orienting shock-sensitive glass plates in ultra clean rooms
ActiveCN101842301ADetailed descriptionConveyorsSemiconductor/solid-state device manufacturingClassical mechanicsEngineering
Disclosed are a method and an apparatus for orienting thin glass plates (11) in a contamination-free manner. Said method and apparatus have the following features: a) in order to orient the glass plate (11), a lifting frame (1) is raised from below through the free space between the rolls (13) along with an orienting frame (2) which rests on the lifting frame (1) and is fitted with struts (7) that are connected in a rotatably hinged fashion to the orienting frame (2) and are provided with supporting elements (8) which penetrate the free space between the rolls (13), project beyond the supporting level of the rolls (13), and support the glass plate (11); b) the glass plate (11) is placed in a shock-free manner by means of sliding elements (3) that are actuated by individually controllable drive units (4), the movement of the sliding elements (3) being transmitted to the two longitudinal cross beams of the orienting frame (2) by means of pivot joints (6) which are fastened to the sliding elements (3).
Owner:GRENZEBACH MASCHINENBAU GMBH
Cleanroom filter unit
InactiveUS6273926B1Simple designCheap productionCombination devicesMechanical apparatusSealantMechanical engineering
A filter unit for airtight attachment to a framework of a cleanroom ceiling includes a filter, a circumferential wall surrounding the filter, and a downwardly projecting sealing knife to be received in a sealant containing groove formed in said framework. The sealing knife includes vertical weldable wall elements interconnected by welding and mounted on the circumferential wall by airtight mounting means.
Owner:ABB FLAEKT AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com