Nifedipine sustained release tablet and preparation method thereof
A nifedipine and gentle technology, which is applied in the field of pharmaceutical and chemical engineering, can solve the problems of dissolution rate difference, insufficient release rate, and drug release that cannot meet clinical needs, and achieve good stability of preparations, reduced number of administrations, and good release curves Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
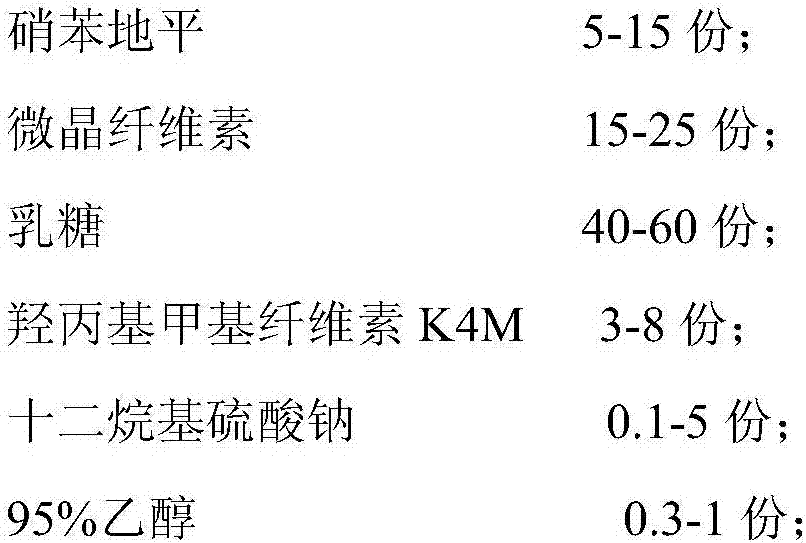
[0030] A nifedipine sustained-release tablet, consisting of the following raw materials in parts by weight:
[0031]
[0032] The method for making described nifedipine slow-release tablet, described method may further comprise the steps:
[0033] Step 1 ingredients: weighing various raw materials;
[0034] Step 2 Granulation: dry mix the prepared raw and auxiliary materials in a mixing granulator for 3 minutes, add a binder to make a soft material, and granulate with a 24-mesh sieve;
[0035] Step 3 drying: place the prepared wet granules in a high-efficiency boiling dryer and dry them at a constant temperature for 20-minutes;
[0036] Step four granulation and total mixing: the dried granules are sieved through a 24-mesh sieve for granulation, add talcum powder and mix in a three-dimensional motion mixer for 15 minutes;
[0037] Step 5 compressing tablet, coating;
[0038] Step 6 Aluminum-plastic packaging, outer packaging, and storage.
[0039] Preferably, in the thi...
Embodiment 2
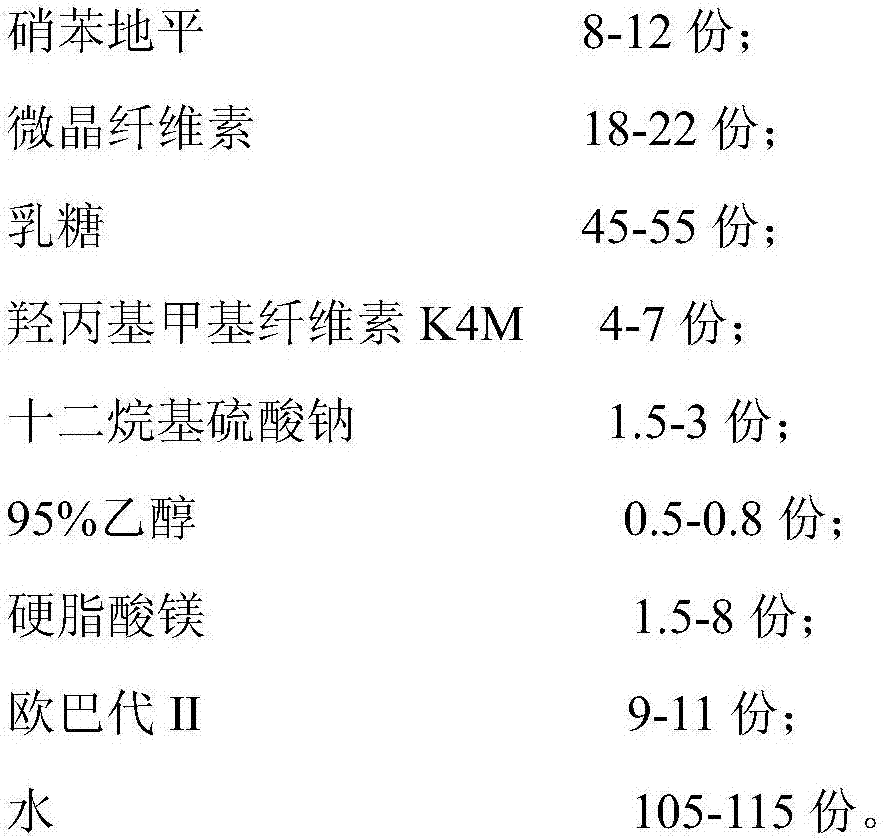
[0043] A nifedipine sustained-release tablet, consisting of the following raw materials in parts by weight:
[0044]
[0045] The method for making described nifedipine slow-release tablet, described method may further comprise the steps:
[0046] Step 1 ingredients: weighing various raw materials;
[0047] Step 2 Granulation: dry mix the prepared raw and auxiliary materials in a mixing granulator for 3 minutes, add a binder to make a soft material, and granulate with a 24-mesh sieve;
[0048] Step 3 Drying: Dry the prepared wet granules in a high-efficiency boiling dryer at a constant temperature for 30 minutes;
[0049] Step four granulation and total mixing: the dried granules are sieved through a 24-mesh sieve for granulation, add talcum powder and mix in a three-dimensional motion mixer for 15 minutes;
[0050] Step 5 compressing tablet, coating;
[0051] Step 6 Aluminum-plastic packaging, outer packaging, and storage.
[0052] Preferably, in the third step, the mo...
Embodiment 3
[0056] A nifedipine sustained-release tablet, consisting of the following raw materials in parts by weight:
[0057]
[0058] The method for making described nifedipine slow-release tablet, described method may further comprise the steps:
[0059] Step 1 ingredients: weighing various raw materials;
[0060] Step 2 Granulation: dry mix the prepared raw and auxiliary materials in a mixing granulator for 3 minutes, add a binder to make a soft material, and granulate with a 24-mesh sieve;
[0061] Step 3 Drying: Dry the prepared wet granules in a high-efficiency boiling dryer at a constant temperature for 25 minutes;
[0062] Step four granulation and total mixing: the dried granules are sieved through a 24-mesh sieve for granulation, add talcum powder and mix in a three-dimensional motion mixer for 15 minutes;
[0063] Step 5 compressing tablet, coating;
[0064] Step 6 Aluminum-plastic packaging, outer packaging, and storage.
[0065] Preferably, in the third step, the mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com