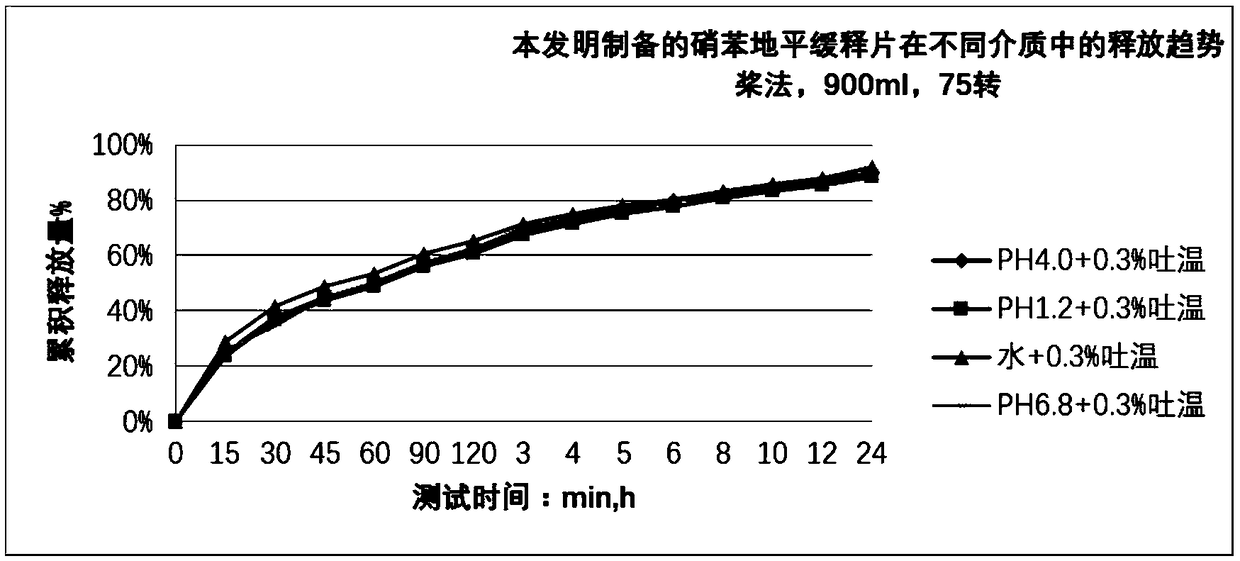
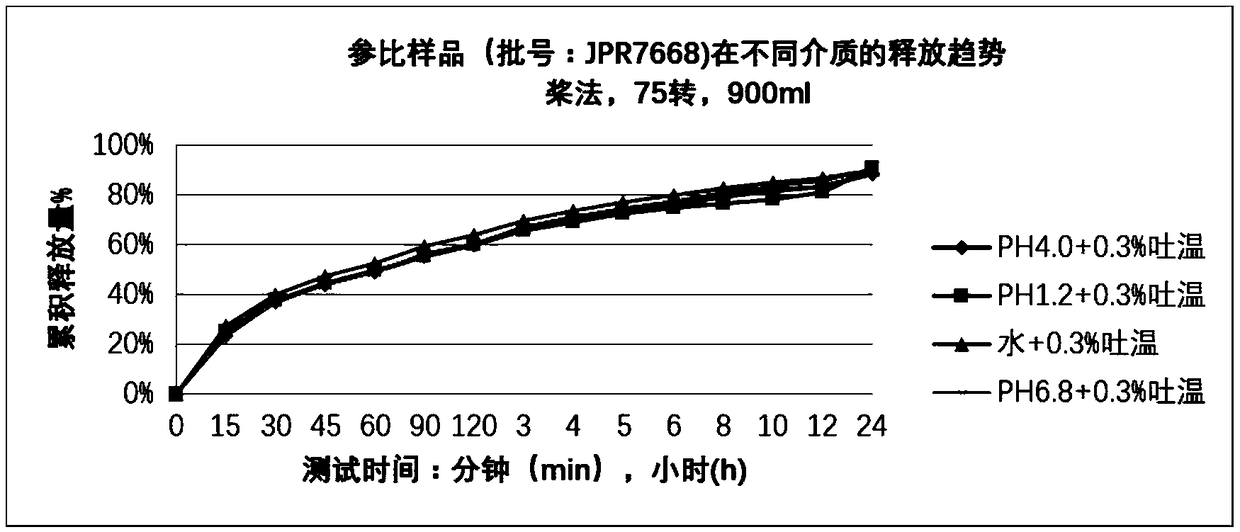
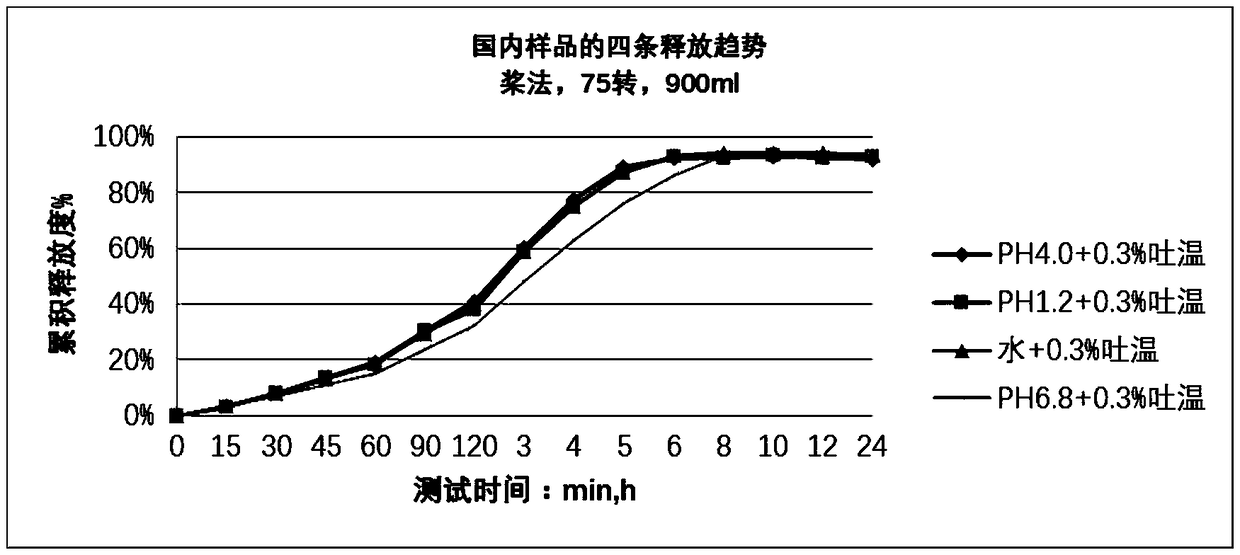
Nifedipine sustained release tablet and preparation method thereof
A technology of nifedipine and nifedipine, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, pill delivery, etc. It can solve problems such as differences in dissolution rate, lack of indicators such as release rate, and drug release that cannot meet clinical needs. , to achieve the effects of good preparation stability, good release curve, and maintenance of drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The nifedipine sustained-release tablet of the present invention is composed of a rapid release component A, a supplementary release component B, a sustained release component C and an auxiliary material magnesium stearate (the content of the following components is in g);
[0043] Expressed in parts by weight, the raw material composition of the quick release component A is: 6g of nifedipine, 10g of microcrystalline cellulose, 4g of lactose hydrate, 0.9g of Tween-80 and 6.5g of purified water;
[0044] Expressed in parts by weight, the raw material composition of the supplementary release component B is: 6g of nifedipine, 5g of microcrystalline cellulose, 4g of lactose hydrate, 5g of starch and 6.0g of purified water;
[0045] Expressed in parts by weight, the raw material composition of the slow-release component C is: 8 g of nifedipine, 28 g of microcrystalline cellulose and 1.85 g of ethyl cellulose;
[0046] The dosage of the added auxiliary material magnesium stea...
Embodiment 2
[0049] The preparation method of the embodiment of the present invention 1 nifedipine slow-release tablet, the detailed steps of this preparation method are as follows:
[0050] a. Preparation of quick release component A:
[0051] First, various raw materials were weighed according to the composition of the quick-release component A described in Example 1, and the weighed Tween-80 was dissolved in purified water to obtain an aqueous solution (1); then the remaining other raw and auxiliary materials were fully mixed to obtain a mixture ( 2); Fully mix the aqueous solution (1) and the mixture (2), and use a wet mixing granulator to make a soft material after mixing; use a 20-mesh nylon sieve to granulate the prepared soft material, and granulate the material obtained after granulation Dry at 55°C in the dark, and sieve the dried material with a 20-mesh nylon sieve to obtain the quick-release component A;
[0052] b. Preparation of supplementary release component B:
[0053]Fi...
Embodiment 3
[0060] The nifedipine sustained-release tablet of the present invention is composed of a rapid release component A, a supplementary release component B, a sustained release component C and an auxiliary material magnesium stearate (the content of the following components is in g);
[0061] Expressed in parts by weight, the raw material composition of the quick release component A is: 30g of nifedipine, 50g of microcrystalline cellulose, 20g of lactose hydrate, 4.5g of Tween-80 and 32.5g of purified water;
[0062] Expressed in parts by weight, the raw material composition of the supplementary release component B is: 30g of nifedipine, 25g of microcrystalline cellulose, 20g of lactose hydrate, 25g of starch and 30.0g of purified water;
[0063] Expressed in parts by weight, the raw material composition of the slow-release component C is: 40 g of nifedipine, 140 g of microcrystalline cellulose and 9.25 g of ethyl cellulose;
[0064] The dosage of the added auxiliary material magn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com