Nifedipine controlled release composition and preparation method thereof
A technology of composition and nitrobenzene, which is applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of unsatisfactory stable release and high cost, achieve a good release curve, prevent product aging, and stabilize formulations Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
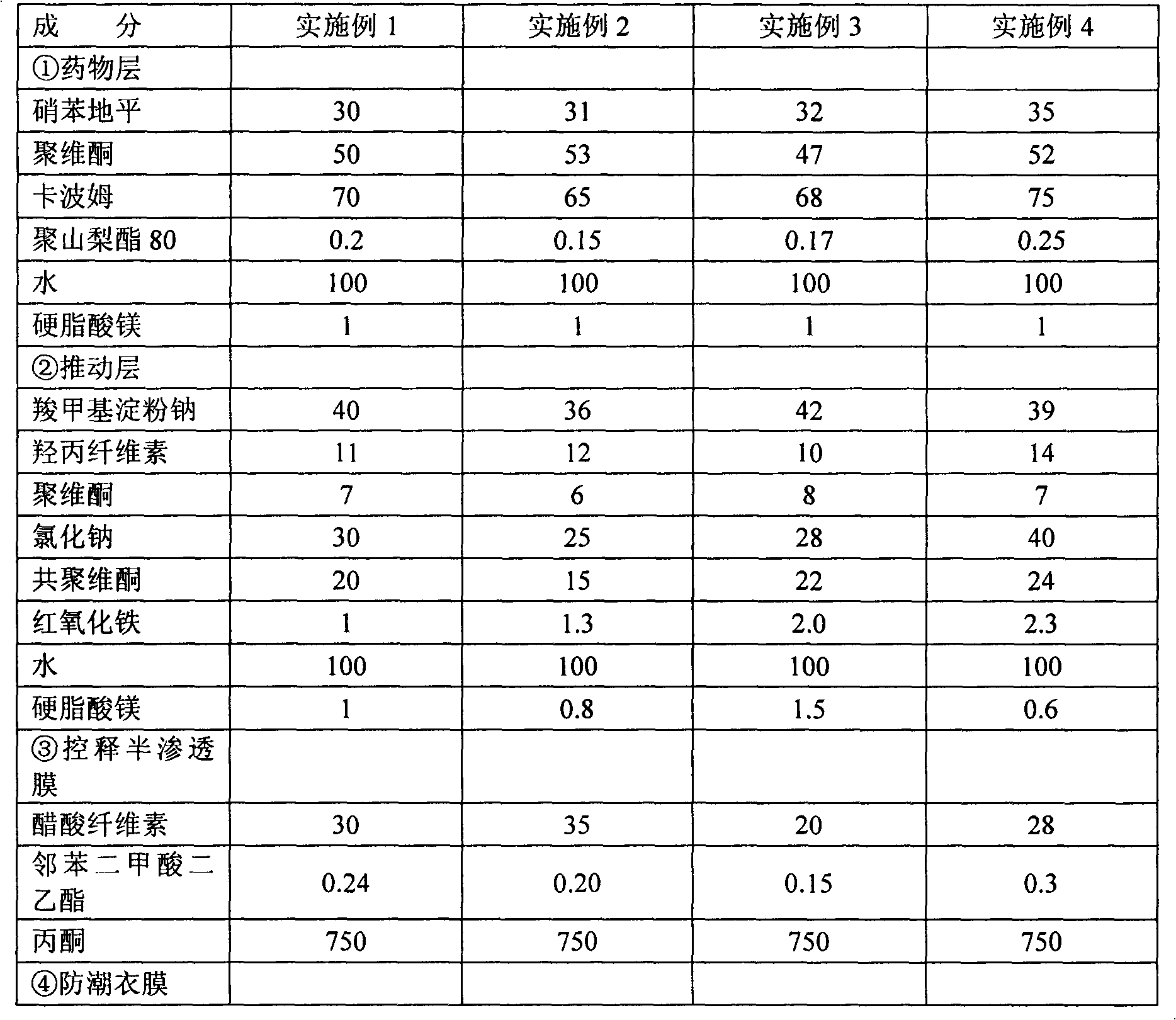
[0028] Weigh each raw material according to the amount in Table 1.
[0029] 1) Drug layer granulation: This step is carried out in the dark, first pass the components of the above drug layer through a 60-mesh sieve, then mix nifedipine, povidone and carbomer evenly, and add them to the fluidized bed middle; spray the above-mentioned amount of polysorbate 80 aqueous solution to granulate; increase the bed temperature to dry; after drying, the granules pass through a 20-mesh sieve for granulation; then add the above-mentioned amount of magnesium stearate, mix well, and set aside;
[0030] 2) Push layer granulation: first pass each component of the above push layer through a 60-mesh sieve, and then press sodium carboxymethyl starch, hypromellose, carbomer, sodium chloride, copovidone and red iron oxide Mix the prescription amount evenly, add to the fluidized bed; spray into purified water to granulate; increase the bed temperature to dry; pass the dried granules through a 20-mesh...
Embodiment 2-4
[0036] Except that the amount of each raw material is weighed according to Table 1, other method steps are all the same as in Example 1. The nifedipine controlled release compositions of Examples 2-4 were prepared.
[0037] Table 1
[0038]
[0039]
[0040] Wherein, all numerical values are the dosage of nifedipine per 1000 tablets, and the unit is g.
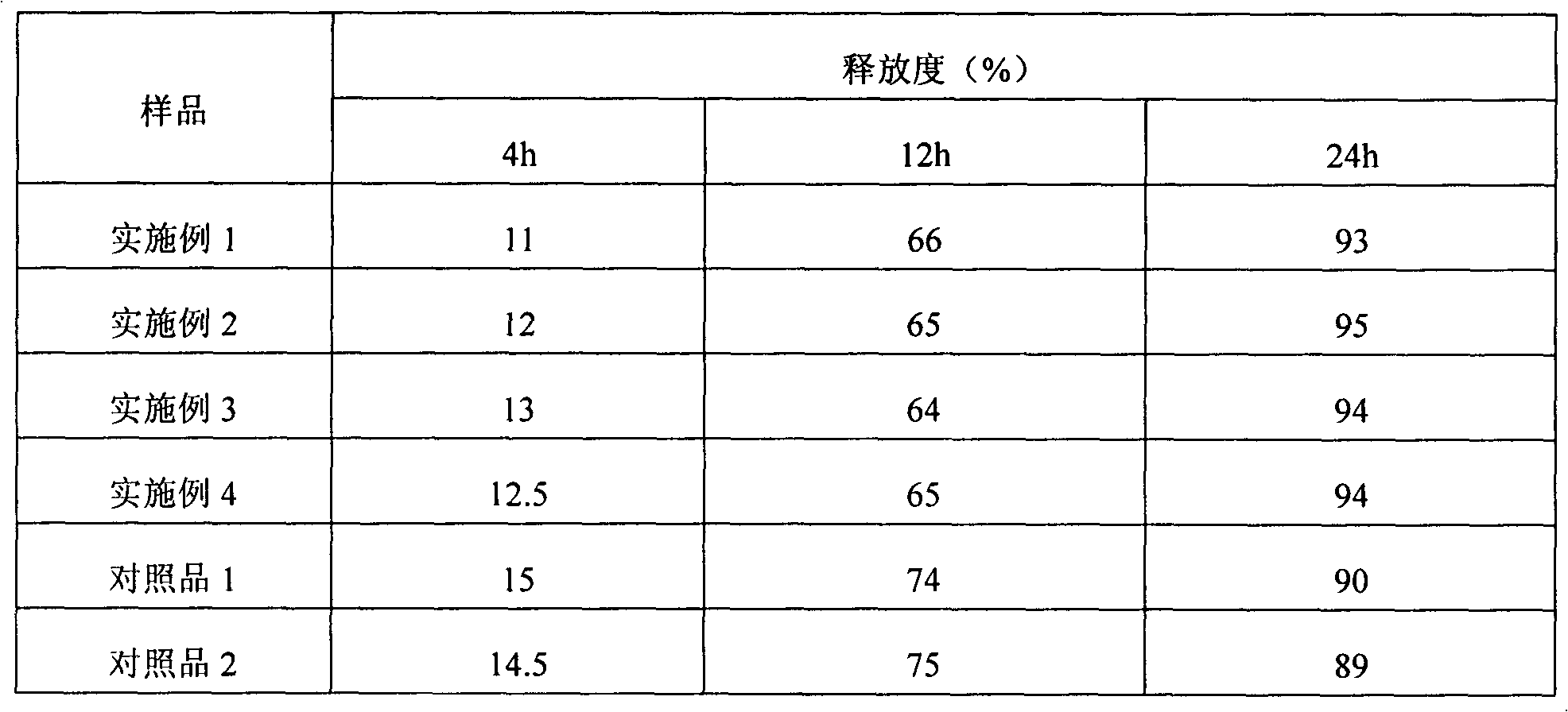
[0041] In addition, commercially available Baixintong was purchased as the reference substance 1, and an asymmetric sheet was made according to Example 2 of CN102151253A as the reference substance 2.
[0042] Test product performance through experiments.
[0043] (1) Content uniformity
[0044] Content uniformity can be used to determine the extent to which a single dose of drug content deviates from the labeled amount. Each tablet of this product contains 30 mg of nifedipine. According to the regulations of the Chinese Pharmacopoeia 2010 edition, there is no need to check the content uniformity. However, since thi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com