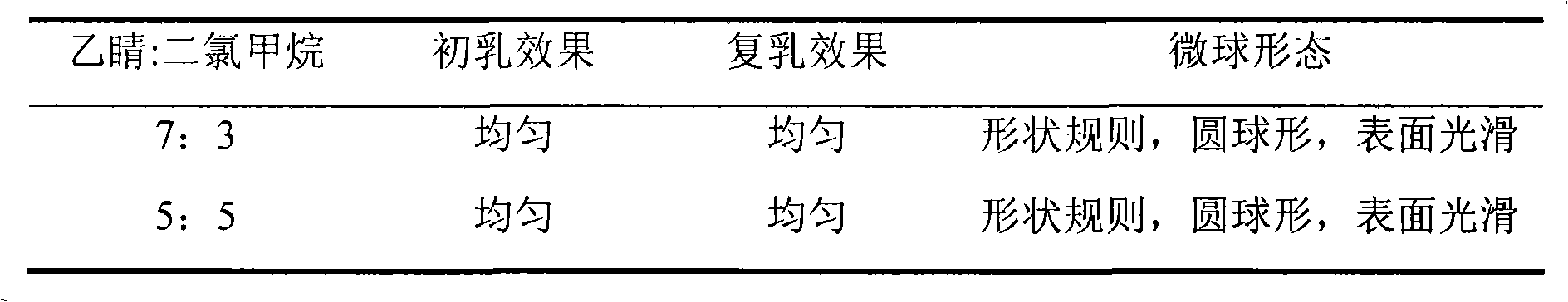
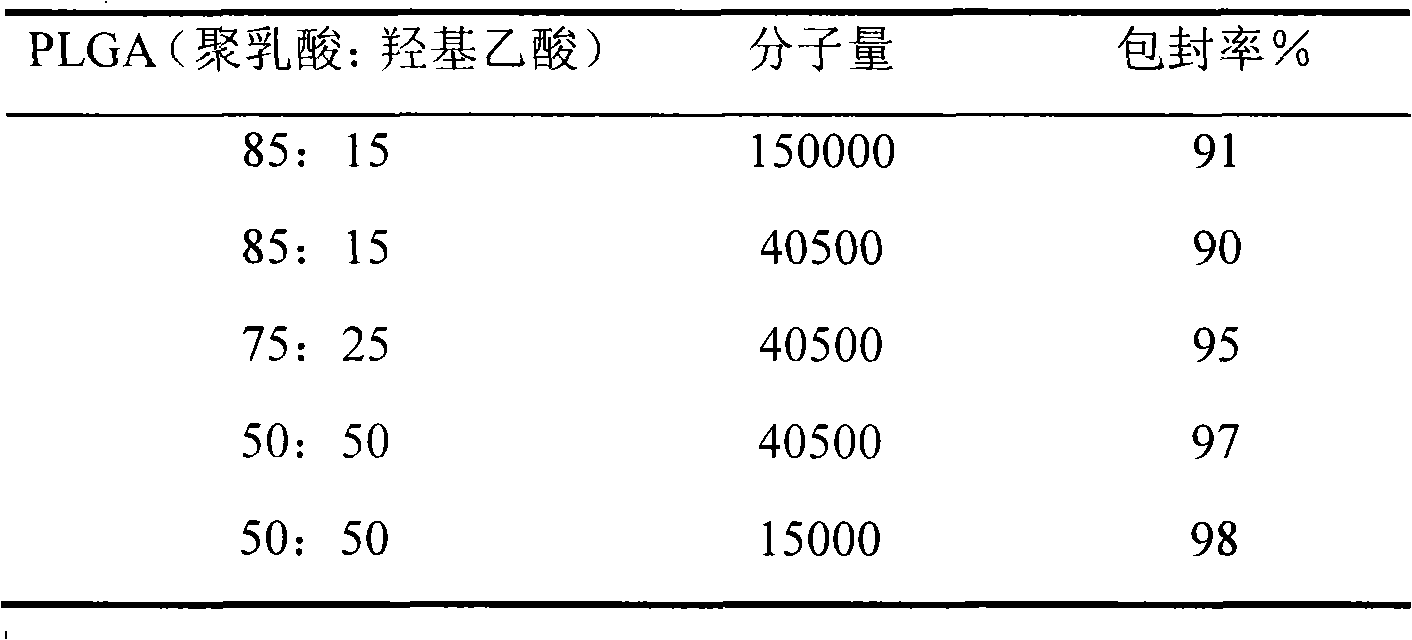
Recombined human vascellum esoderma inhibin durative action preparation, preparation method and application thereof
A vascular endothelium and statin technology, applied in the field of medicine, can solve problems such as pain of patients, and achieve the effects of reducing pain, reducing burden and improving curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Dissolve 100mg PLGA (Mw=15000, 50:50) in 1ml 1-methyl-2-pyrrolidone (concentration is 10%), then add 0.1ml 100mg / ml Endostar aqueous solution (the weight ratio of PLGA to Endostar is 10: 1) Mix with a shaker for 30 minutes, add the mixed solution to a dialysis bag with a molecular weight cut-off of 30,000 and a diameter of 1 cm with a syringe, and tighten with clamps at both ends. Then place the dialysis bag in a beaker containing 10 ml of phosphate buffer (0.1 mol / L, pH 7.0), and keep stirring with a magnetic stirrer. The solvent is rapidly dialyzed into the beaker and the PLGA solidifies to form the implanted pump. Take 1ml of the buffer solution in the beaker every day, and replenish the same volume of fresh phosphate buffer solution at the same time. The content of Endostar in the samples was determined by HPLC. The cumulative release percentage of the drug was plotted against the release time. 18.8% was released on day 1, 55.5% was released on day 7, 58.0% was r...
Embodiment 2
[0047] Dissolve 100mg PLGA (Mw=40500, 50:50) in 1ml 1-methyl-2-pyrrolidone (concentration is 10%), then add 0.1ml 200mg / ml Endostar aqueous solution (the weight ratio of PLGA and Endostar is 10 : 2), all the other steps are with embodiment 1. The cumulative release percentage of the drug was plotted against the release time. 18.1% was released on day 1, 21.5% was released on day 7, 51.0% was released on day 14, and 89.1% was released on day 28.
Embodiment 3
[0049] Dissolve 100mg of PLGA (Mw=40500, 75:25) in 1ml of 1-methyl-2-pyrrolidone (concentration is 10%), then add 0.05ml of 100mg / ml Endostar aqueous solution (the weight ratio of PLGA to Endostar is 20 : 1), all the other steps are with embodiment 1. The cumulative release percentage of the drug was plotted against the release time. 7.6% was released on day 1, 11.5% on day 7, 23.0% on day 14, and 54.1% on day 28.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com