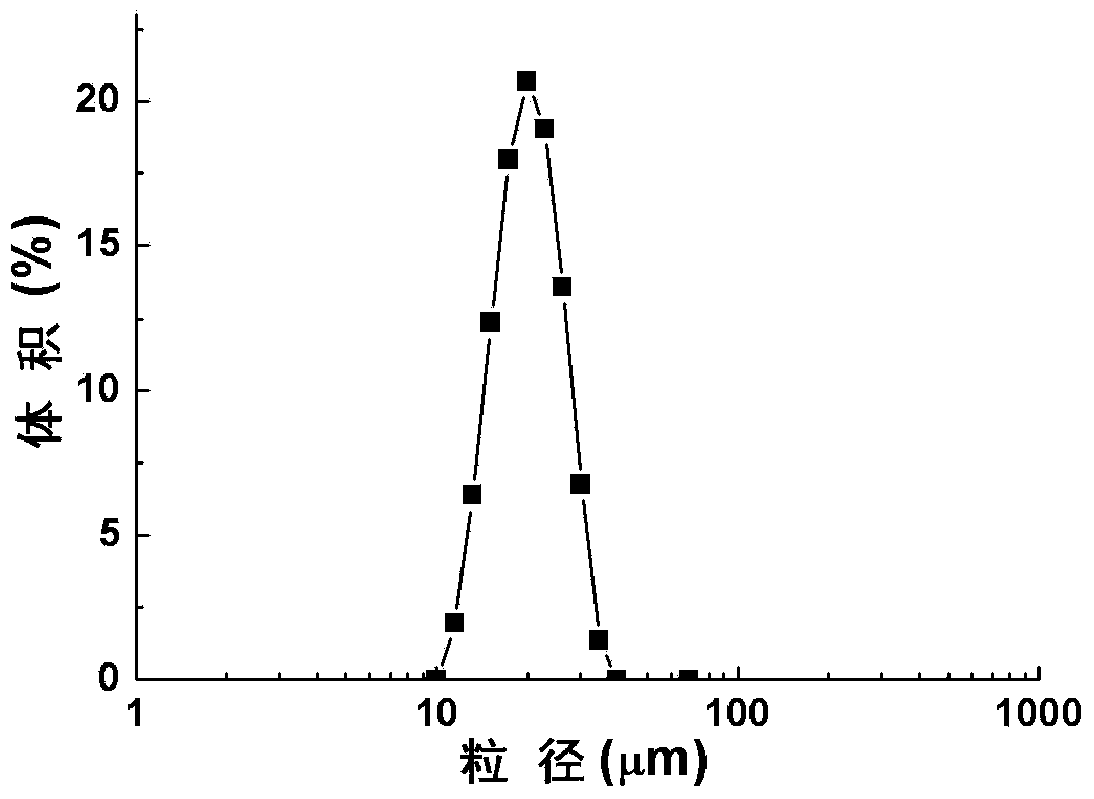
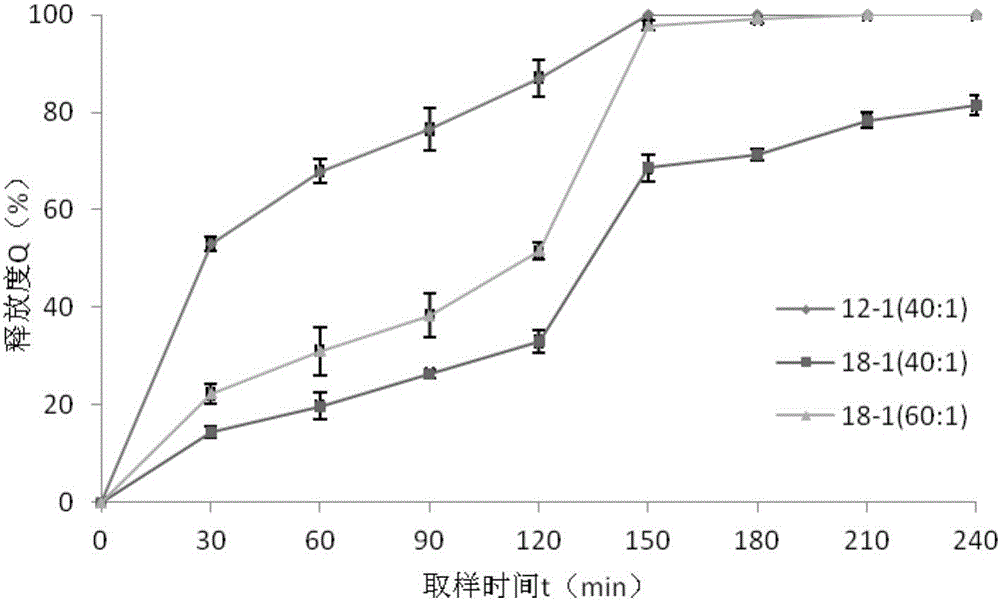
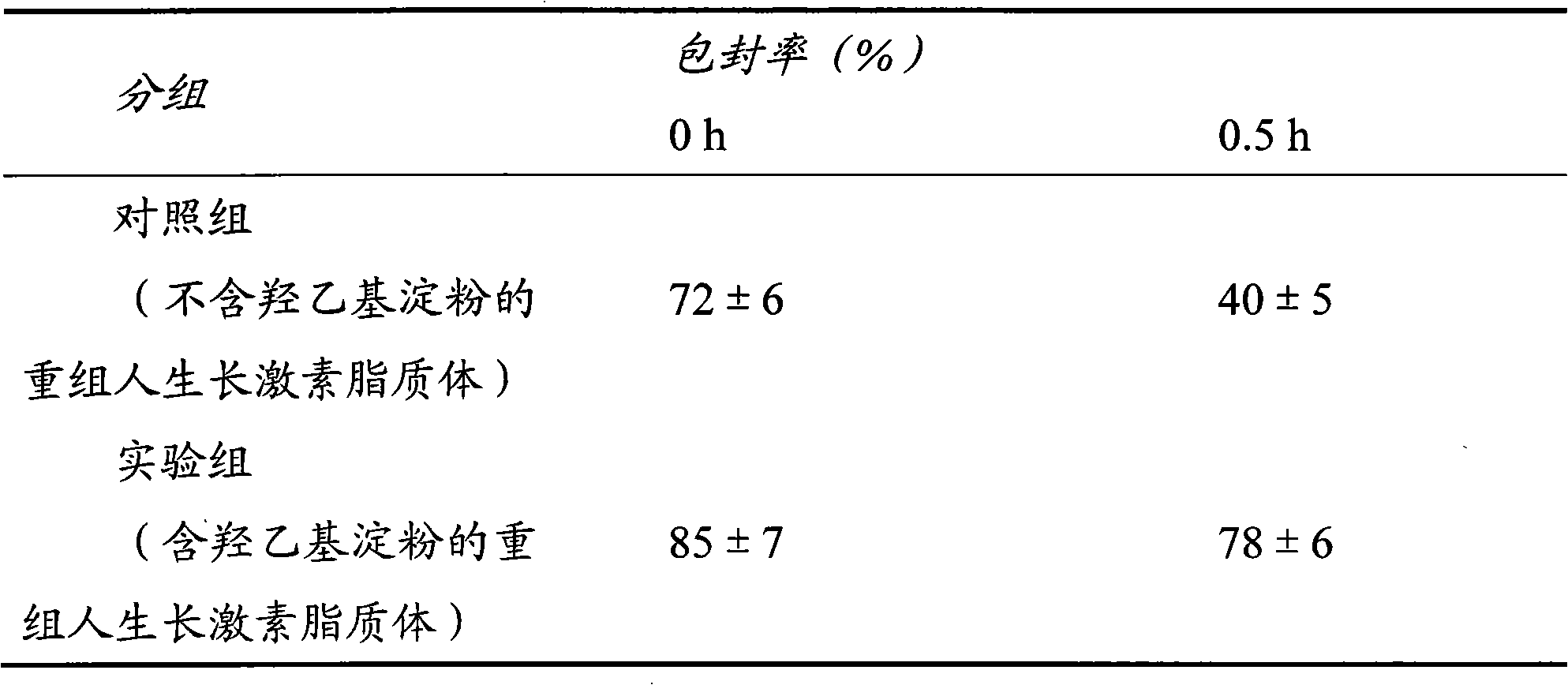
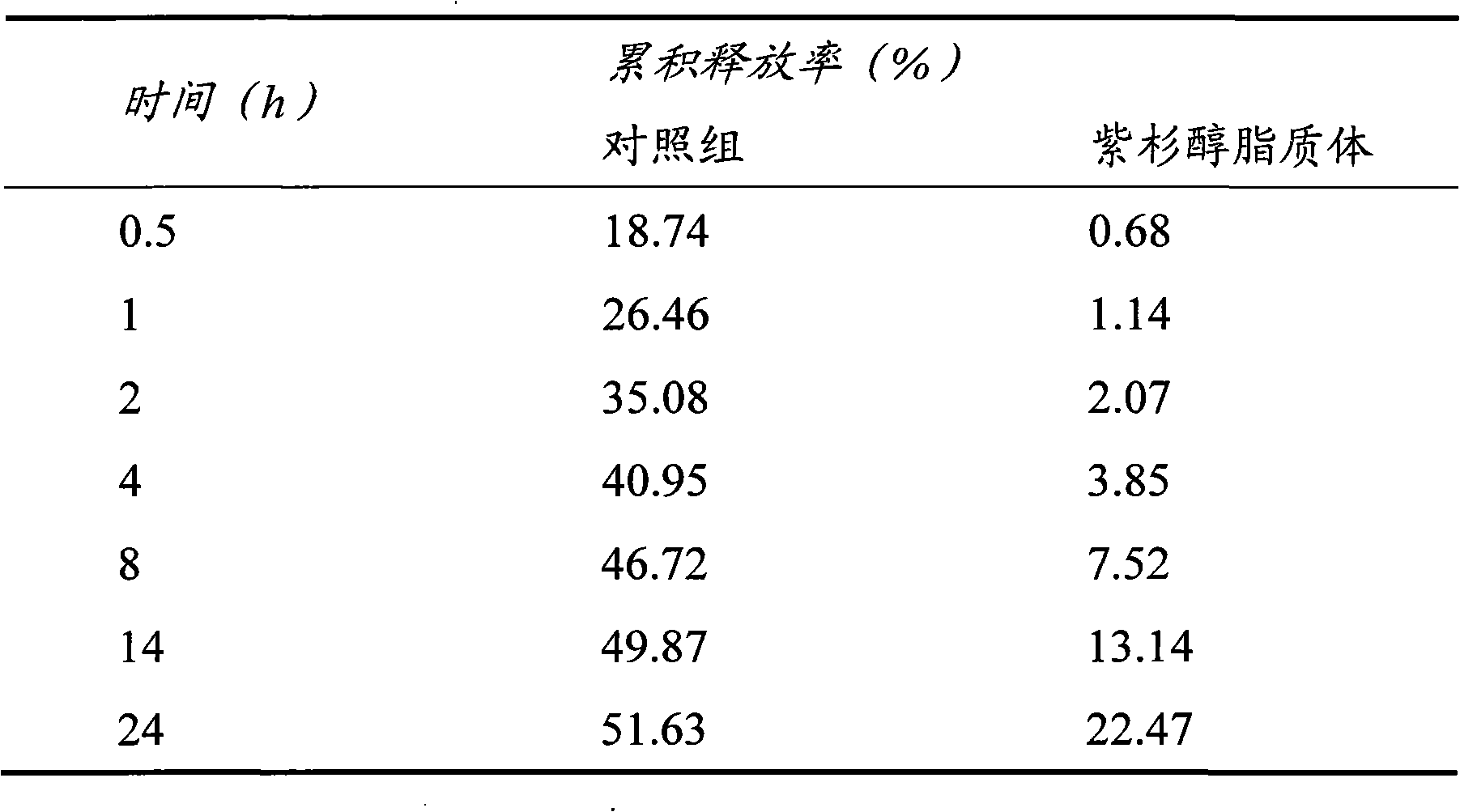
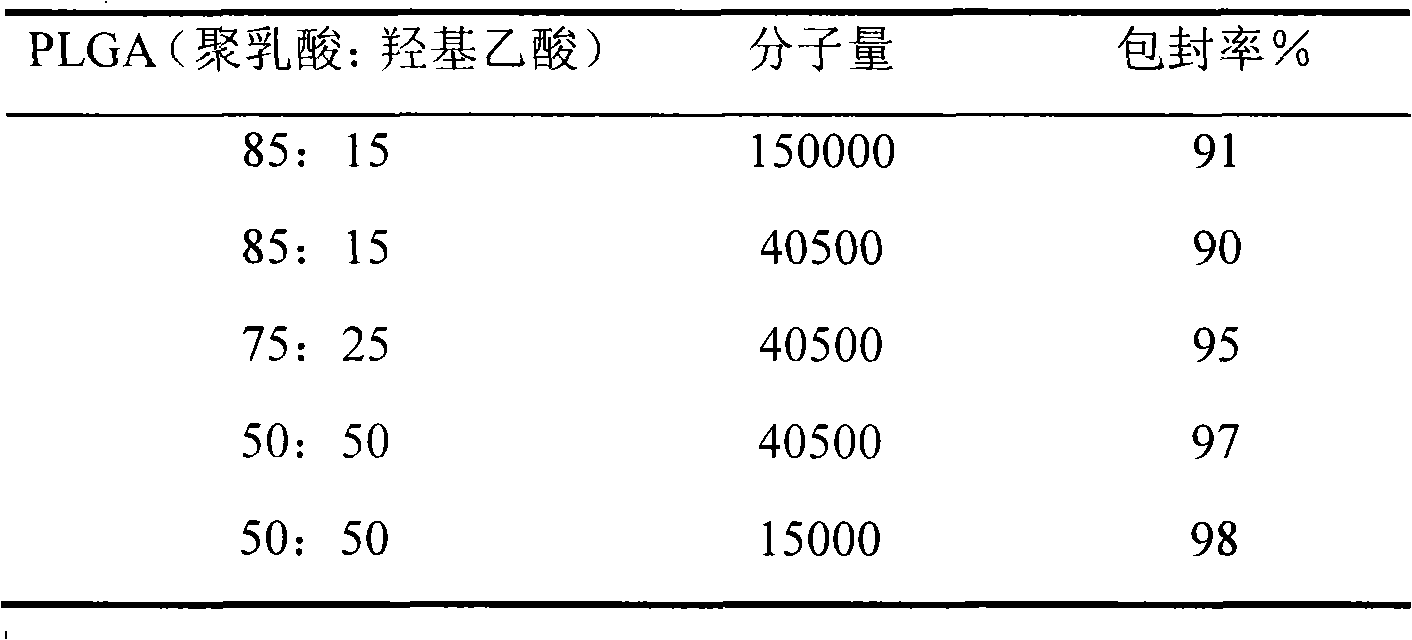
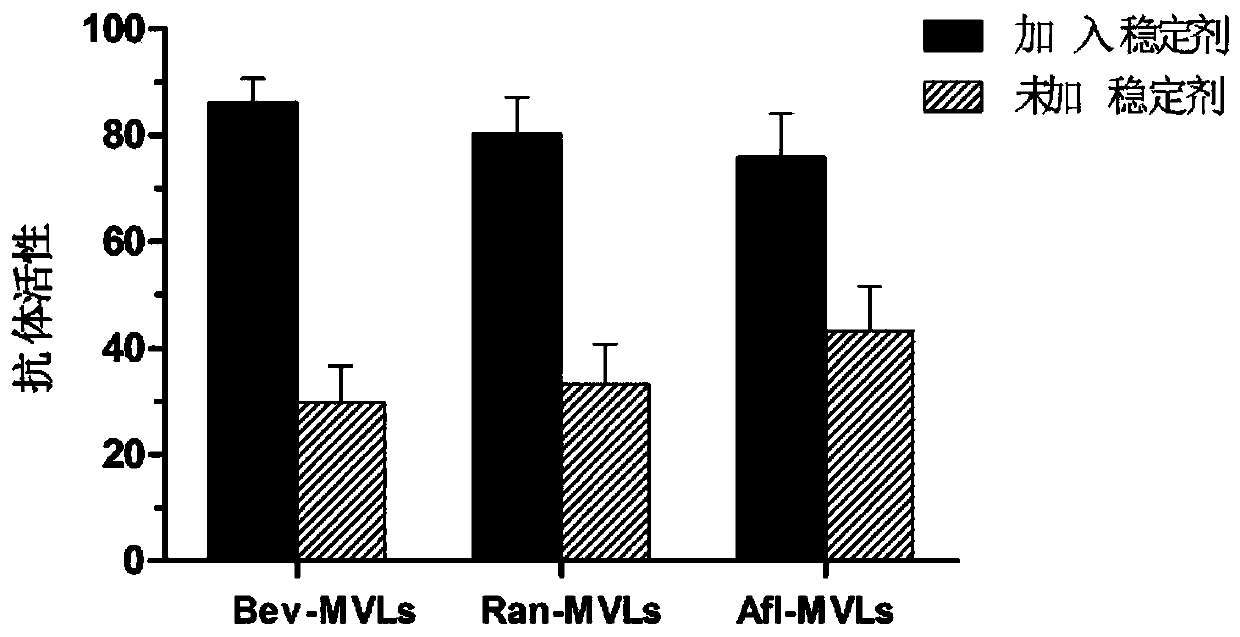
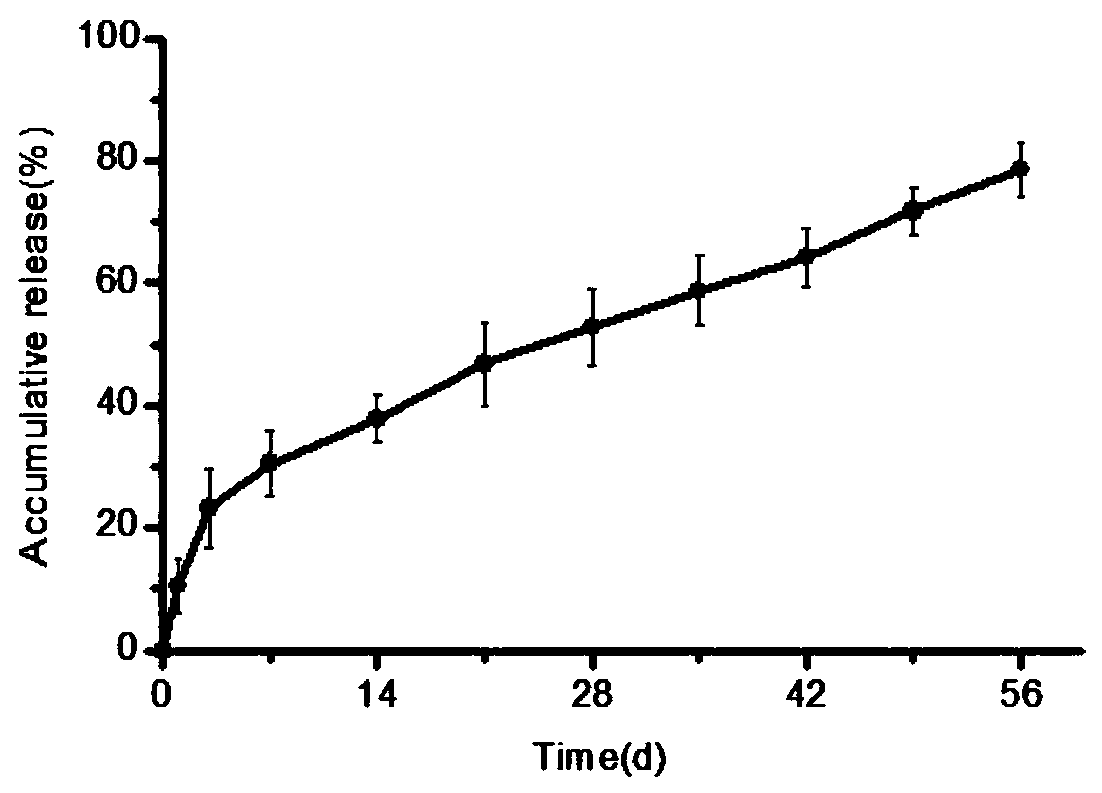
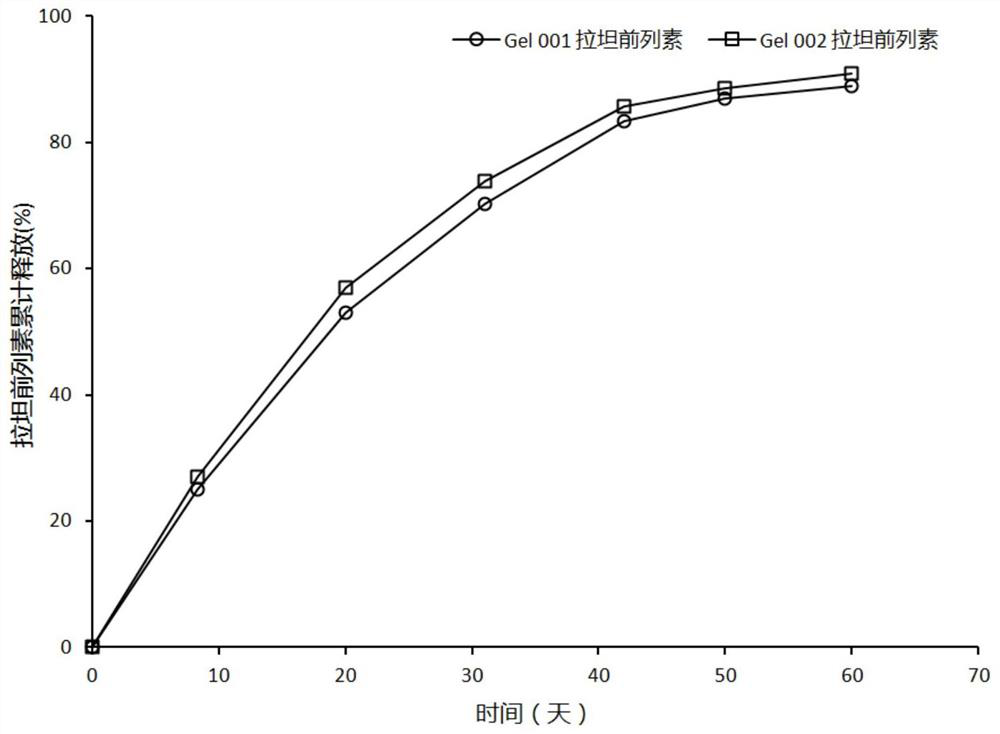
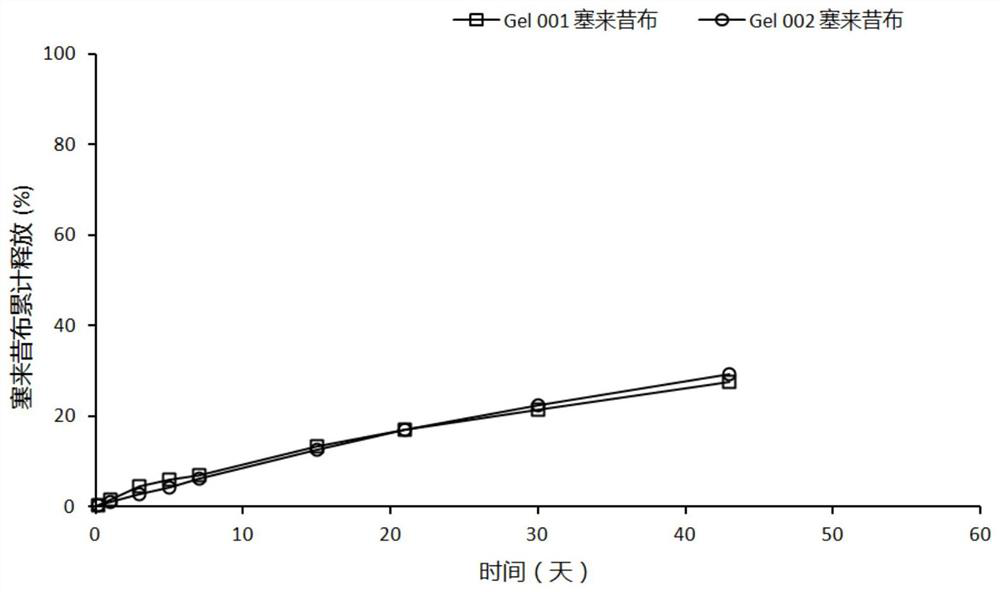
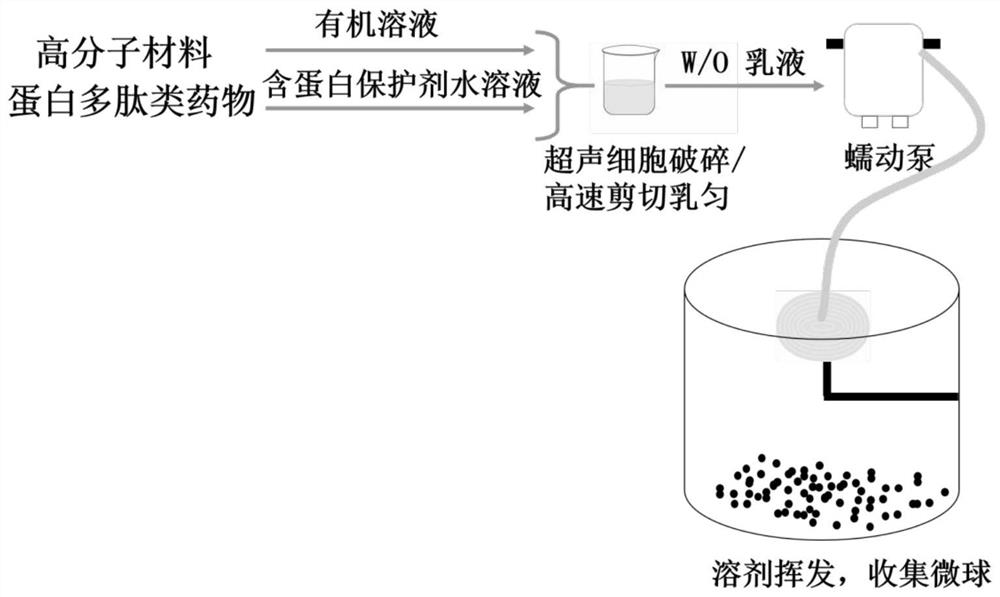
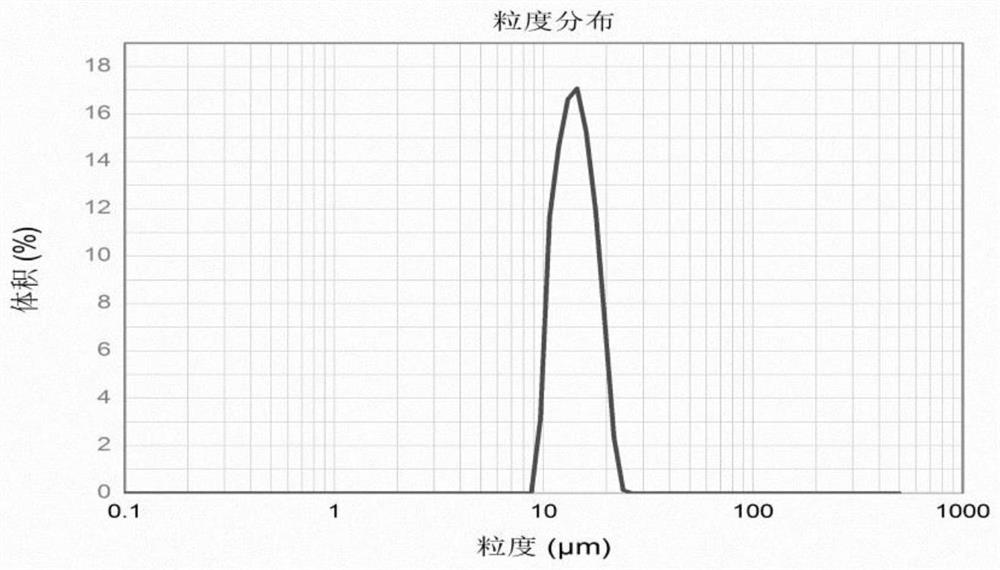
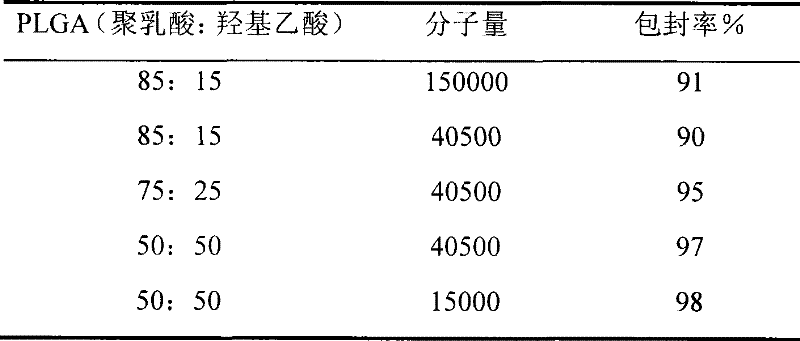
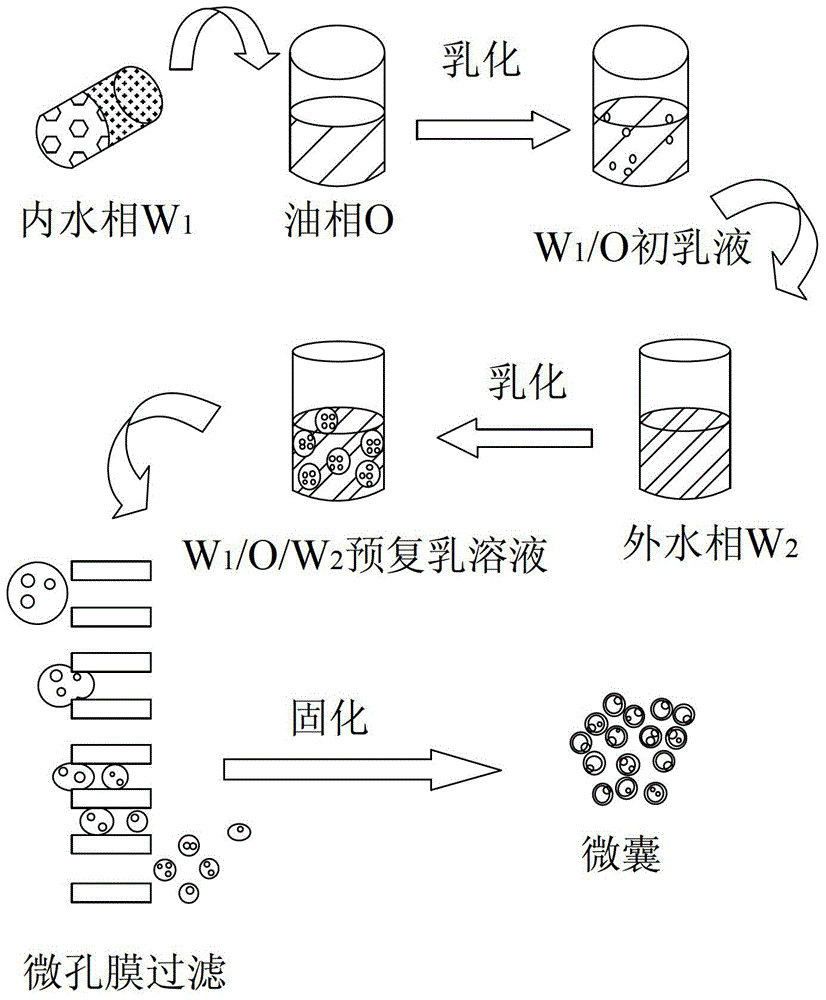
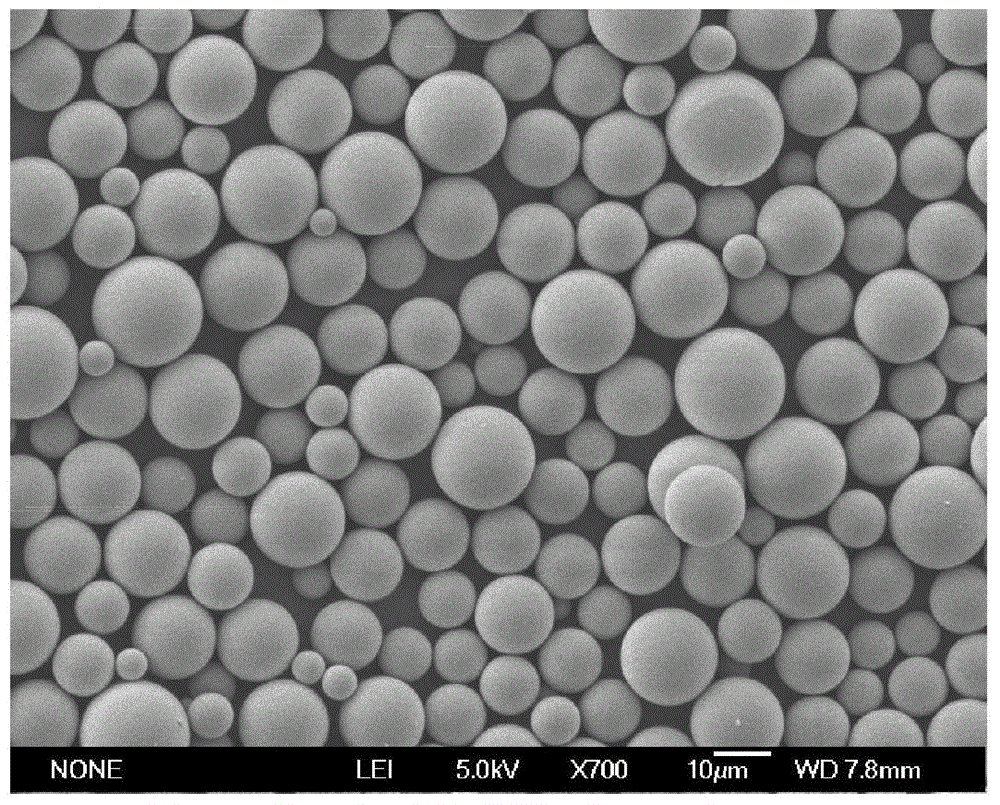
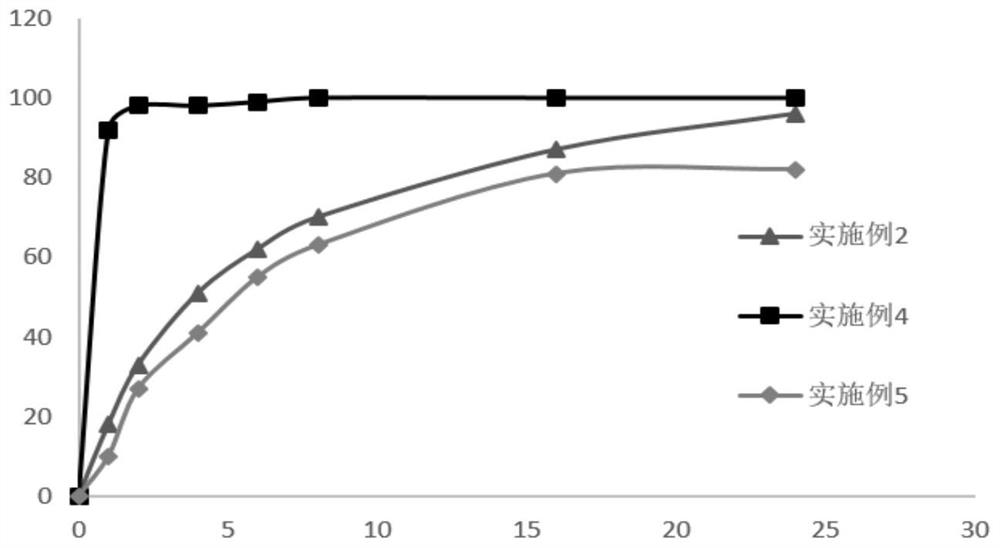
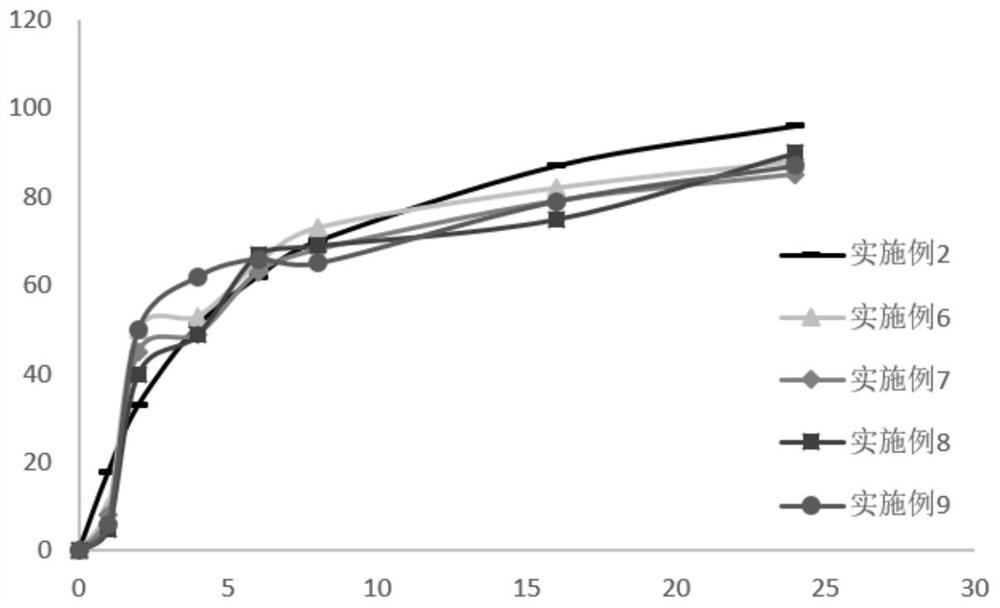
Patents
Literature
33results about How to "Reduce burst phenomenon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ternary composite microsphere formulation and its preparation method
InactiveCN1557283AReduce incomplete releaseImprove protectionPharmaceutical delivery mechanismMicroballoon preparationLactideMicrosphere
The triple composite microsphere preparation consists of mainly model medicine, calcium alginate, chitosan, and diglycolide-lactide copolymer. The preparation process includes W / O emulsification, washing with isopropanol to prepare small calcium alginate microcapsule, coating with chitosan to form compact double-layered alginic acid-chitonsan microcapsule, the subsequent emulsifying and solvent volatilization step to coat the double-layered alginic acid-chitonsan microcapsule into diglycolide-lactide copolymer to form the three-layered composite microsphere. The present invention can protect protein and polypeptide medicine in hydrophilic sodium alginate-chitonsan microcapsule environment to reduce abrupt release and incomplete release, prolong the medicine release time, and regulate the medicine releasing mode via altering PLGA composition.
Owner:ZHEJIANG UNIV
Small molecule hydrophilic drug-embedded sustained-release capsule and preparation method thereof
ActiveCN104224753AReduce spreadHigh embedding ratePharmaceutical delivery mechanismMacromolecular non-active ingredientsSolventOil phase
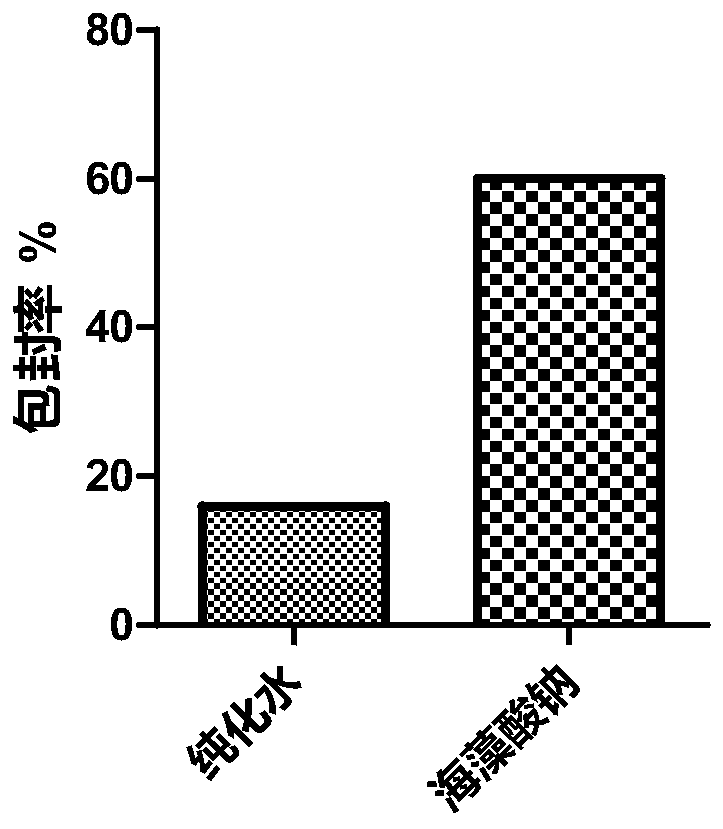
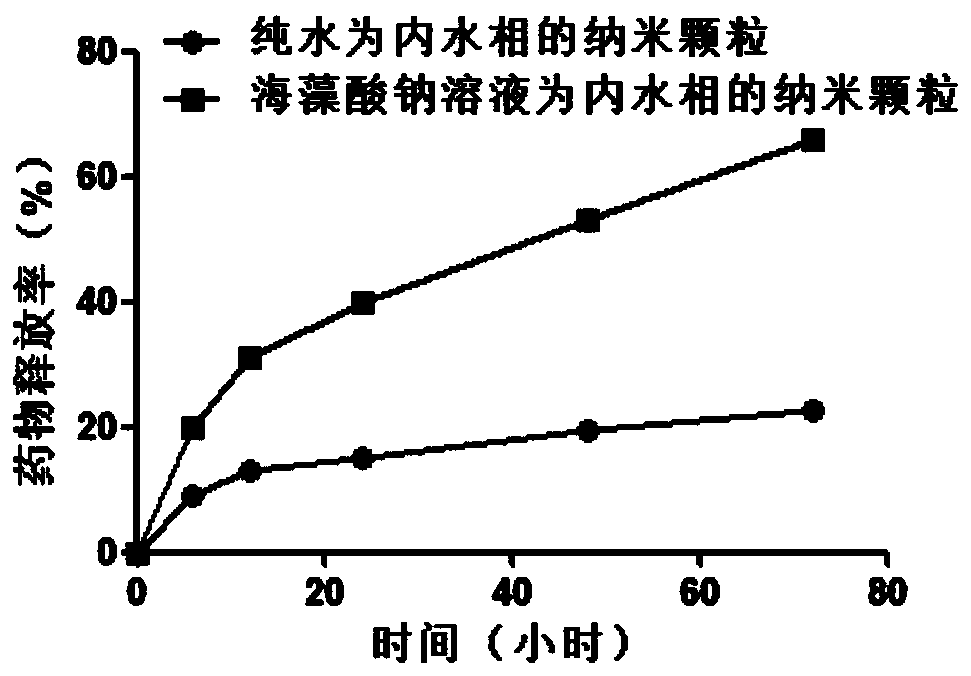
The invention relates to a method for preparing a small molecule hydrophilic drug-embedded sustained-release capsule. The method comprises the following steps: filtering a prepared'internal aqueous phase / oil phase / external aqueous phase' pre-multi-emulsion solution through a microporous membrane; and then removing a solvent, washing and drying to obtain the small molecule hydrophilic drug-embedded sustained-release capsule, wherein the internal aqueous phase comprises a small molecule hydrophilic drug and a thickening agent. The small molecule hydrophilic drug-embedded sustained-release capsule provided by the invention has the advantages of uniform grain diameter, high embedding rate, low burst-release rate, stable drug release and long action.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for compounding and entrapping tea polyphenols through debranched starch and xanthan gum
InactiveCN106509899AShort half-lifeFast digestion and absorptionFood ingredientsFood shapingHydrolysateIon exchange
The invention relates to a method for compounding and entrapping tea polyphenols through debranched starch and xanthan gum. The method comprises the following steps of (1) making starch raw materials into starch milk and performing sufficient gelatinizing; (2) lowering the temperature to the appropriate temperature of debranching enzymes, adding the debranching enzymes for enzymolysis, and then performing ion exchange, decoloring and concentration on enzymatic hydrolysate; (3) enabling the xanthan gum to dissolve in the water, and performing compounding with the concentrated enzymatic hydrolysate of the debranched starch; (4) maintaining the temperature of a compounding system solution to be 70-80 DEG C, maintaining the temperature to be the temperature range, under the stirring condition, adding the tea polyphenols, and performing uniform stirring; and (5) uniformly stirring the solution, then sending the uniformly-stirred solution into a high-pressure homogenizer for homogenizing for several times, then sending the homogenized solution into a spray drying system, and under the condition of being away from light, performing stirring and spray drying at the same time so as to obtain tea polyphenol microcapsules. The method is simple to operate, the starch is used as a main raw material, and through compounding with the xanthan gum, so that the stable state of the tea polyphenols is realized; and the tea polyphenols can be slowly released in the digestive tract of human bodies, so that the bioavailability of the tea polyphenols is improved, and the application range of the starch is extended.
Owner:JIANGNAN UNIV
Drug-loaded liposome and preparation method thereof
ActiveCN101926770APrevent flocculationInhibit aggregationMacromolecular non-active ingredientsAntineoplastic agentsHydroxyethyl starchFlocculation
The invention relates to a drug-loaded liposome and preparation method thereof. The liposome contains hydrogenated lecithin or synthetic phospholipid composition and hydroxyethyl starch assuring stable structure of the liposome. The liposome is used to encapsulate and load drugs, so as to control flocculation, agglomeration, precipitation and the like of the liposome, reduce leakage of the encapsulated and loaded drugs, prolong the release of drugs, improve stability of liposome preparation in liquid and solid states; therefore, it is convenient for the storage and the transportation of the preparation. The liposome is suitable for encapsulating various drugs, and is especially suitable for insoluble drugs, heat-reactive drugs and biomacromolecule drugs, and the fitting range is wide.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Phospholipid protein particle composite microsphere and preparation method thereof
ActiveCN105616385AReduce drug burstProtect biological activityPeptide/protein ingredientsPharmaceutical non-active ingredientsPhospholipid transfer proteinSolvent
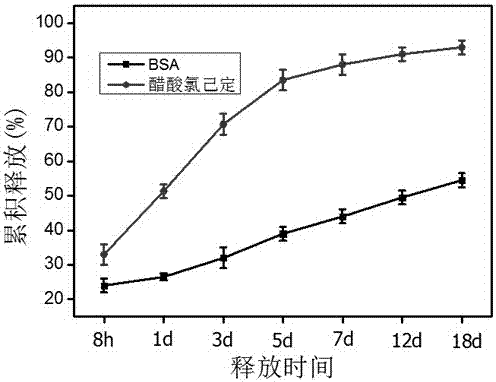
The invention relates to a phospholipid protein particle composite microsphere and a preparation method thereof. The preparation method comprises the following steps: stirring and mixing protein or polypeptide water-soluble drugs, an aqueous solution of a freeze-drying protective additive and an alcoholic solution of phospholipid to obtain a lipid vesicle suspension of phospholipid protein; freeze-drying for removing the solvent to obtain phospholipid protein particles; uniformly dispersing the phospholipid protein particles into an organic solution of a polymer carrier material, then adding an aqueous solution containing an emulsifying agent, and shearing at a high speed to prepare emulsion of S / O / W; and carrying out the processes of solvent volatilization, microsphere curing and the like to form the phospholipid protein particle composite microsphere. The phospholipid protein particle composite microsphere prepared by the method provided by the invention has a high drug envelopment rate and a low sudden release rate, wherein the release rate at the first day is 9-15%; the release rate is stable and lasting, the slow release period of the preparation can reach 20-60 days, and the bioactivity of the drugs in the microsphere is high; and therefore, the phospholipid protein particle composite microsphere has practical values in clinical application.
Owner:SUN YAT SEN UNIV
Colon-targeted gel microsphere with controllable core-shell ratio, and preparation and application of colon-targeted gel microsphere
ActiveCN113384557AReduce releaseReduce burst phenomenonAerosol deliveryDigestive systemMicrosphereActive matter
The invention provides a colon-targeted gel microsphere with a controllable core-shell ratio, and preparation and application of the colon-targeted gel microsphere. The gel microsphere comprises a core layer, an inner shell layer and an outer shell layer from inside to outside in sequence, wherein the core layer comprises cereal prolamin and an active matter; the inner shell layer comprises gel polysaccharide; the outer shell layer comprises chitosan, a cross-linking agent and a suspending aid. The inner shell layer and the core layer form core-shell structure fog drops through a coaxial electrostatic spraying technology, in an outer shell layer solution, the inner shell layer gel polysaccharide of the core-shell structure fog drops is cross-linked with the cross-linking agent and is subjected to electrostatic layer-by-layer adsorption with chitosan, and therefore, the colon-targeted gel microsphere is prepared. The particle size of the gel microsphere is 100-600 [mu] m, the core-shell ratio is 0.5-0.9, the encapsulation efficiency of a water-soluble active matter is as high as 60%, the encapsulation efficiency of an alcohol-soluble active matter is as high as 90%, the release amounts in a stomach simulation liquid and a small intestine simulation liquid within 1 h and 3 h can be as low as 10% and 25%, and the gel microsphere can be efficiently delivered and targeted to the colon.
Owner:SOUTH CHINA AGRI UNIV
Antibacterial and wound healing promoting composition and medical hydrocolloid oil yarn thereof
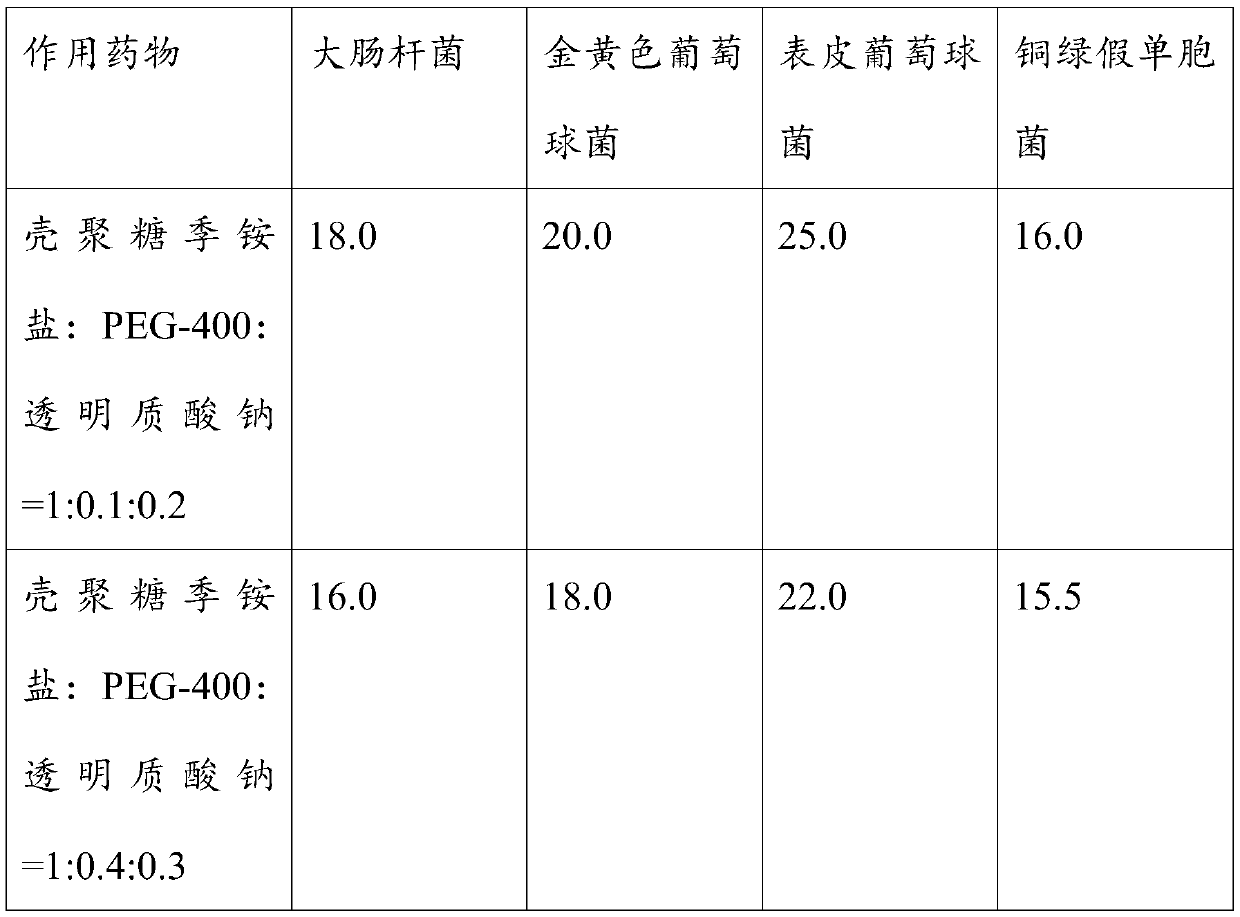
ActiveCN110974999AHigh antibacterial activityIncrease local concentrationAbsorbent padsBandagesMicrospherePolyethylene glycol
The invention relates to the field of medicines, and provides an antibacterial and wound healing promoting composition and a medical hydrocolloid oil yarn thereof. The composition capable of resistingbacteria and promoting wound healing comprises chitosan quaternary ammonium salt, polyethylene glycol and sodium hyaluronate with a mass ratio of 1:(0.1-0.6):(0.2-0.4). The antibacterial and wound healing promoting composition is embedded by using gelatin to form microspheres, and the microspheres are added into a hydrocolloid oil yarn to form the medical hydrocolloid oil yarn which has the effects of inhibiting gram-negative bacteria and gram-positive bacteria and also has the remarkable effects of diminishing inflammation, promoting wound healing and repairing. Compared with the prior art,remarkable progress is achieved.
Owner:GUANGZHOU RAINHOME PHARM&TECH CO LTD
Protein polypeptide drug long-acting microspheres and preparation method thereof
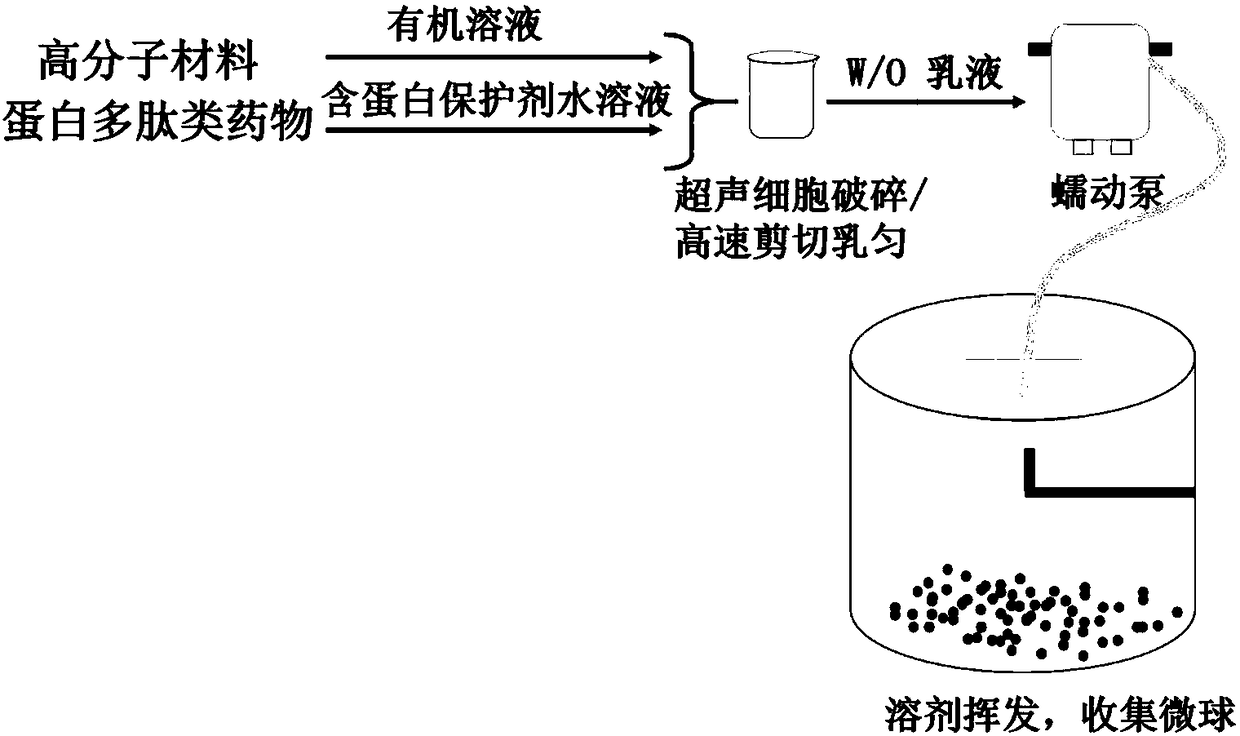
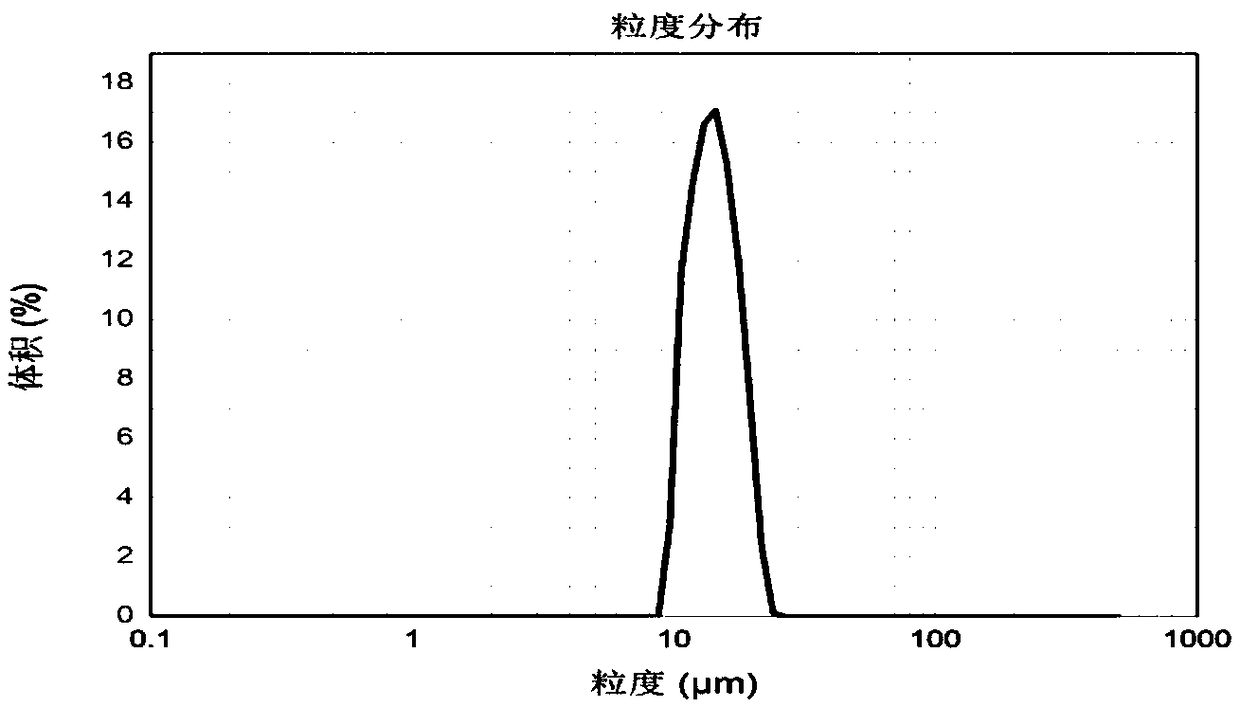
ActiveCN108403643AEasy to prepareMild preparation methodPeptide/protein ingredientsPharmaceutical non-active ingredientsDrugs solutionPeristaltic pump
The invention relates to protein polypeptide drug long-acting microspheres and a preparation method thereof. The preparation method comprises the steps of (1) dissolving a protein or polypeptide water-soluble drug and a protein protectant to obtain an aqueous drug solution; dissolving a macromolecular carrier material in an organic solvent to obtain an oil phase macromolecular solution; (2) addingthe aqueous drug solution into the oil phase macromolecular solution, and using a cell disrupter or a high-speed shear apparatus to prepare W / O emulsion; (3) feeding the W / O emulsion into a superfineparticle preparation system through a peristaltic pump so as to obtain the protein polypeptide drug long-acting microspheres. The protein polypeptide drug long-acting microspheres prepared via the preparation method have the advantages of good preparation process simplicity, controllable parameters, good reproducibility, and high production efficiency. The protein polypeptide drug long-acting microspheres prepared herein have the advantages of high drug encapsulating rate, low sudden release agent, stable and lasting release speed of drug, smooth microsphere surface, good spherical shape integrity, and homogenous particle size.
Owner:SUN YAT SEN UNIV
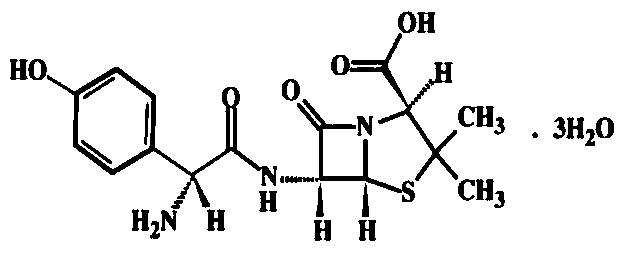
Amoxicillin capsule and preparation method thereof
ActiveCN104856972BHigh dissolution rateReduce volumeAntibacterial agentsUrinary disorderCross-linkDissolution
The invention discloses an amoxicillin capsule and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The components contained in the amoxicillin capsule and their mass fractions are: 100 parts of amoxicillin, 0.1-1 part of sodium lauryl sulfate, 0.1-3 parts of talcum powder, and 0.25-2.0 parts of magnesium stearate. The present invention ensures the higher dissolution rate of amoxicillin by adjusting the particle size of raw material amoxicillin particles and the ratio of auxiliary materials, avoids the use of disintegrants such as sodium carboxymethyl starch and crospovidone, and reduces the amount of amoxicillin The volume of the amoxicillin capsule; the production and configuration steps of the medicine of the amoxicillin capsule are simplified, the production cost is reduced, the content of high molecular impurities in the amoxicillin capsule is reduced, and the production efficiency is improved.
Owner:NORTH CHINA PHARMA COMPANY
Drug-loaded liposome and preparation method thereof
ActiveCN101926770BImprove stabilityNot easily oxidizedMacromolecular non-active ingredientsAntineoplastic agentsHydroxyethyl starchFlocculation
The invention relates to a drug-loaded liposome and preparation method thereof. The liposome contains hydrogenated lecithin or synthetic phospholipid composition and hydroxyethyl starch assuring stable structure of the liposome. The liposome is used to encapsulate and load drugs, so as to control flocculation, agglomeration, precipitation and the like of the liposome, reduce leakage of the encapsulated and loaded drugs, prolong the release of drugs, improve stability of liposome preparation in liquid and solid states; therefore, it is convenient for the storage and the transportation of the preparation. The liposome is suitable for encapsulating various drugs, and is especially suitable for insoluble drugs, heat-reactive drugs and biomacromolecule drugs, and the fitting range is wide.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Microsphere for double protection of antibody drug and intravitreal injection, and preparation method thereof
ActiveCN108938600AGood denaturation effectPromote aggregationSenses disorderAntibody ingredientsSide effectMicrosphere
The invention provides a microsphere for double protection of antibody drug and intravitreal injection, and a preparation method thereof. Particles of a monoclonal antibody drug are prepared by adopting a method of water phase-water phase emulsification, polylactic acid-glycolic acid copolymer and polyketal are utilized as carrier materials, and a sustained release microsphere that wraps and carries the particles of a dextran-monoclonal antibody drug is prepared by employing an emulsion solvent volatilization method of water phase-oil phase-solid phase. Denaturation and aggregation of antibodies are caused by an acidic microenvironment of polylactic acid-glycolic acid in an organic phase / aqueous phase interface and a degradation process, the drug loading capacity of the microsphere is increased and a burst release phenomenon during a releasing process of the microsphere is reduced, and double protection on the monoclonal antibody drug of the microsphere is achieved; the polyketal enables the drug loading capacity of the microsphere to be increased, enables the stimulation of an acidic degradation product of the polylactic acid and glycolic acid on eye environment to be reduce, andenables side effects such as endophthalmitis to be reduced; and the microsphere can release the monoclonal antibody for not less than 28 days in the in vitro environment and for not less than 2 monthsin the eye, and so, the frequency of administration can be reduced, pain of patients and economic burden are reduced, and the compliance of patients is improved.
Owner:南京锐利施生物技术有限公司
Ternary composite microsphere formulation and its preparation method
InactiveCN1268325CReduce incomplete releaseImprove protectionMicroballoon preparationMicrocapsulesLactideMicrosphere
The triple composite microsphere preparation consists of mainly model medicine, calcium alginate, chitosan, and diglycolide-lactide copolymer. The preparation process includes W / O emulsification, washing with isopropanol to prepare small calcium alginate microcapsule, coating with chitosan to form compact double-layered alginic acid-chitonsan microcapsule, the subsequent emulsifying and solvent volatilization step to coat the double-layered alginic acid-chitonsan microcapsule into diglycolide-lactide copolymer to form the three-layered composite microsphere. The present invention can protect protein and polypeptide medicine in hydrophilic sodium alginate-chitonsan microcapsule environment to reduce abrupt release and incomplete release, prolong the medicine release time, and regulate the medicine releasing mode via altering PLGA composition.
Owner:ZHEJIANG UNIV
Colon-targeted sinomenine hydrochloride slow-release nanofiber membrane and preparation method and application thereof
InactiveCN111020880AReduce irritationReduce burst phenomenonOrganic active ingredientsAntipyreticSinomenine hydrochlorideDrug loading dose
The invention discloses a colon-targeted sinomenine hydrochloride slow-release nanofiber membrane and a preparation method and application thereof. The nanofiber membrane comprises a shell layer and acore layer coated in the shell layer, wherein the shell layer comprises the following components: Eudragit S100 or Eudragit RS100; the core layer comprises the following components: any one or more than two of Eudragit S100, Eudragit RS100, polycaprolactone and polyvinylpyrrolidone, and sinomenine hydrochloride. The drug loading capacity of sinomenine hydrochloride in the nanofiber membrane is 10%-30%. According to the sinomenine hydrochloride slow-release nanofiber membrane with the shell-core structure, slow release and targeting of the drug are realized and the membrane has good biocompatibility.
Owner:GUANGXI UNIV FOR NATITIES
Recombined human vascellum esoderma inhibin durative action preparation, preparation method and application thereof
ActiveCN101292951ARelieve painReduce the burden onPeptide/protein ingredientsPharmaceutical delivery mechanismMicrosphereRelease time
The invention relates to the field of medical technology and discloses a long-acting preparation of lactic-glycolic acid copolymer (PLGA) for the recombination of endostatin of vein and the application to preparing a drug for treating tumor. The invention uses four methods of implanting a pump in vivo, implanting a microsphere in vivo, a W / O / W solvent volatilization method and a W / O / O solvent volatilization method to prepare the long-acting preparation. The sustained-release time of the long-acting preparation prepared is 7 to 28 days in accordance with the needs.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
Polycystic liposome gel capable of overcoming burst release and maintaining antibody activity and preparation method thereof
ActiveCN109985236AGood biocompatibilityPromote degradationAerosol deliveryOintment deliveryRetention timeBiocompatibility Testing
The invention discloses a polycystic liposome gel capable of overcoming burst release and maintaining antibody activity, and belongs to the field of eye sustained and controlled release preparations of biological macromolecular drugs. A monoclonal antibody clone antibody drug is wrapped in the polycystic liposome with good biocompatibility and biodegradability, and then the polycysticliposome is dispersed in sol formed by a temperature-sensitive polymer material, and after the sol is injected through a vitreous cavity, the sol is solidified into semi-solid gel at the body temperature to form apolycysticliposome-temperature-sensitive hydrogel composite preparation, so that release of the antibody drug is controlled. By the adoption of the polycystic liposome gel capable of overcoming burstrelease and maintaining antibody activity, the advantages of the polycystic liposome and the hydrogel preparation are utilized, in-vitro release of the antibody drug can be kept for about 60 days, the burst release problem of a general dosage form of the antibody drug is reduced, the activity of the antibody drug is greatly maintained, retention time of the antibody drug in eyes after vitreous injection is prolonged to a greater extent, injection frequency is reduced, and compliance of patients is improved.
Owner:YANTAI UNIV
Composite drug-loaded gel and preparation method thereof
ActiveCN113181108AImprove hydrophilicityGood water dispersibilityOrganic active ingredientsAerosol deliveryPolypyrroleDrug release
The invention provides a composite drug-loaded gel and a preparation method thereof, and the preparation method comprises the following steps: S1, adding pyrrole and dopamine hydrochloride into water, uniformly mixing, then adding ammonium persulfate, carrying out a stirring reaction, and carrying out centrifugal washing to obtain a polypyrrole-polydopamine nano composite material; S2, sequentially putting the nano composite material into a 1mol / L hydrochloric acid solution containing drug molecules and a graphene oxide aqueous solution, and carrying out centrifugal adsorption to obtain a graphene oxide-polypyrrole-polydopamine nano composite drug-loading material; and S3, mixing the nano-composite drug-loading material with a water-soluble polymer solution, adding a calcium chloride solution, stirring, and standing to obtain the composite drug-loading gel. The composite drug-loading material provided by the invention is compounded with a water-soluble polymer, so that the formation of composite drug-loading gel can be promoted, the burst release phenomenon at the initial stage of drug release is slowed down, and meanwhile, the composite drug-loading gel has multiple stimulation responsiveness.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Polypeptide HM-3 nanometer particles and preparation method thereof
PendingCN111467473ANo change in activityDoes not affect particle sizeSenses disorderPeptide/protein ingredientsDiseaseBlood plasma
The invention provides polypeptide HM-3 nanometer particles and a preparation method thereof. The polypeptide HM-3 nanometer particles are characterized by consisting of polypeptide HM-3, sodium alginate, chitosan, a polylactic acid-amino-oxyacetic acid copolymer (PLGA), an emulsifying agent, a pharmaceutic adjuvant and the like. According to the polypeptide HM-3 nanometer particles provided by the invention, the polypeptide HM-3 is packed in an inner aqueous phase, so that the stability of the polypeptide HM-3 is improved, the release of the polypeptide HM-3 is controlled, and the plasma half-life is prolonged. The polypeptide HM-3 nanometer particles provided by the invention can be applied to treatment of diseases associated with blood vessel regeneration, such as tumors, age-related macular degeneration and the like.
Owner:CHINA PHARM UNIV
Preparation method of fish collagen double sustained-release film
InactiveCN103705936AGood biocompatibilityPromote degradationHydroxy compound active ingredientsMacromolecular non-active ingredientsWhole bodyBiotin
The invention relates to a fish collagen double sustained-release film and a preparation method thereof. An anti-tumor drug is encapsulated by biotin-coupled cholesteric glycol chitosan self-aggregated nanoparticles, and then drug-loaded nanoparticles are compounded with waste fish skin collagen and chitosan, so as to prepare the fish collagen double sustained-release film in manners of freezing and drying. The film has the advantages that local slow release is carried out, toxic and side effects of the whole body are reduced, and the like.
Owner:FUZHOU UNIV
Composite microspheres for ordered release of growth factors and antibiotics, preparation method and application
InactiveCN105030699BReduce burst phenomenonGuaranteed efficacyAntibacterial agentsPowder deliveryDiseaseMicrosphere
Owner:FUZHOU UNIV
Phospholipid protein particle composite microsphere and preparation method thereof
ActiveCN105616385BImprove biological activityReduce biological activityPeptide/protein ingredientsPharmaceutical non-active ingredientsFreeze-dryingMicrosphere
Owner:SUN YAT SEN UNIV
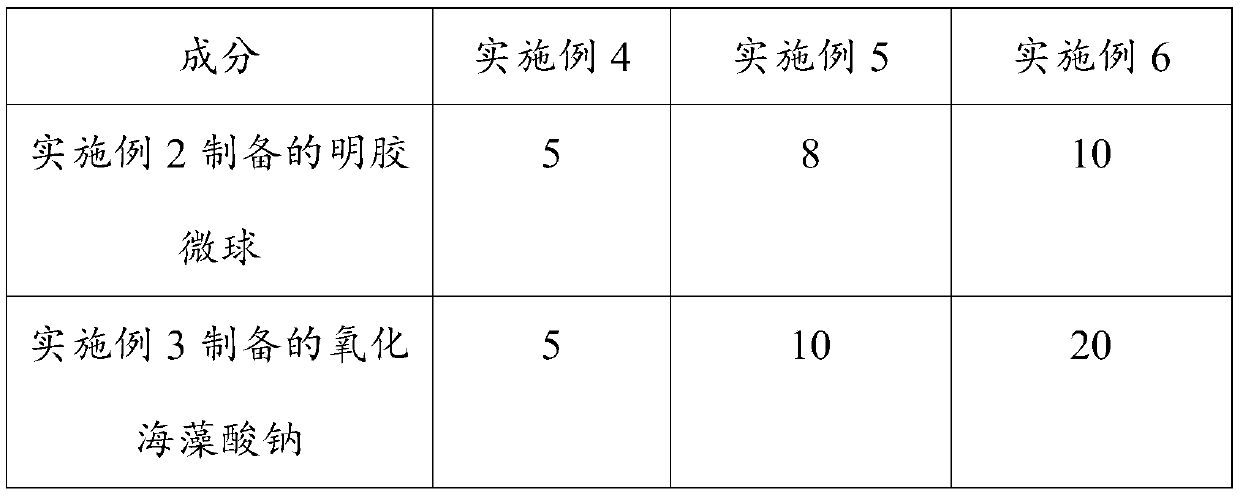
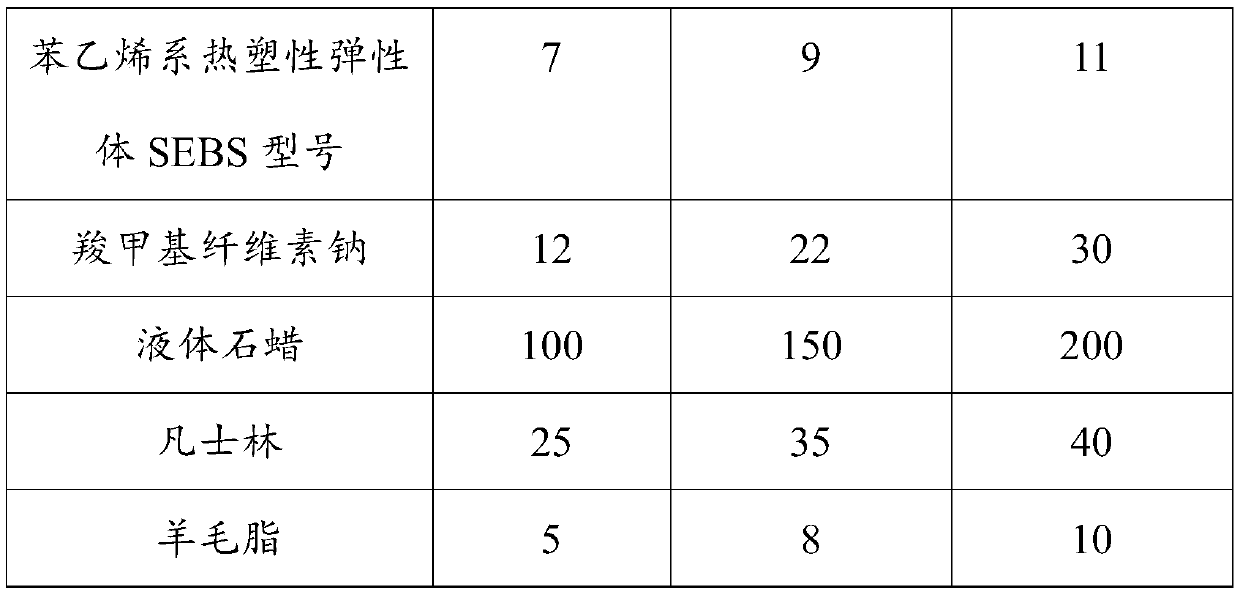
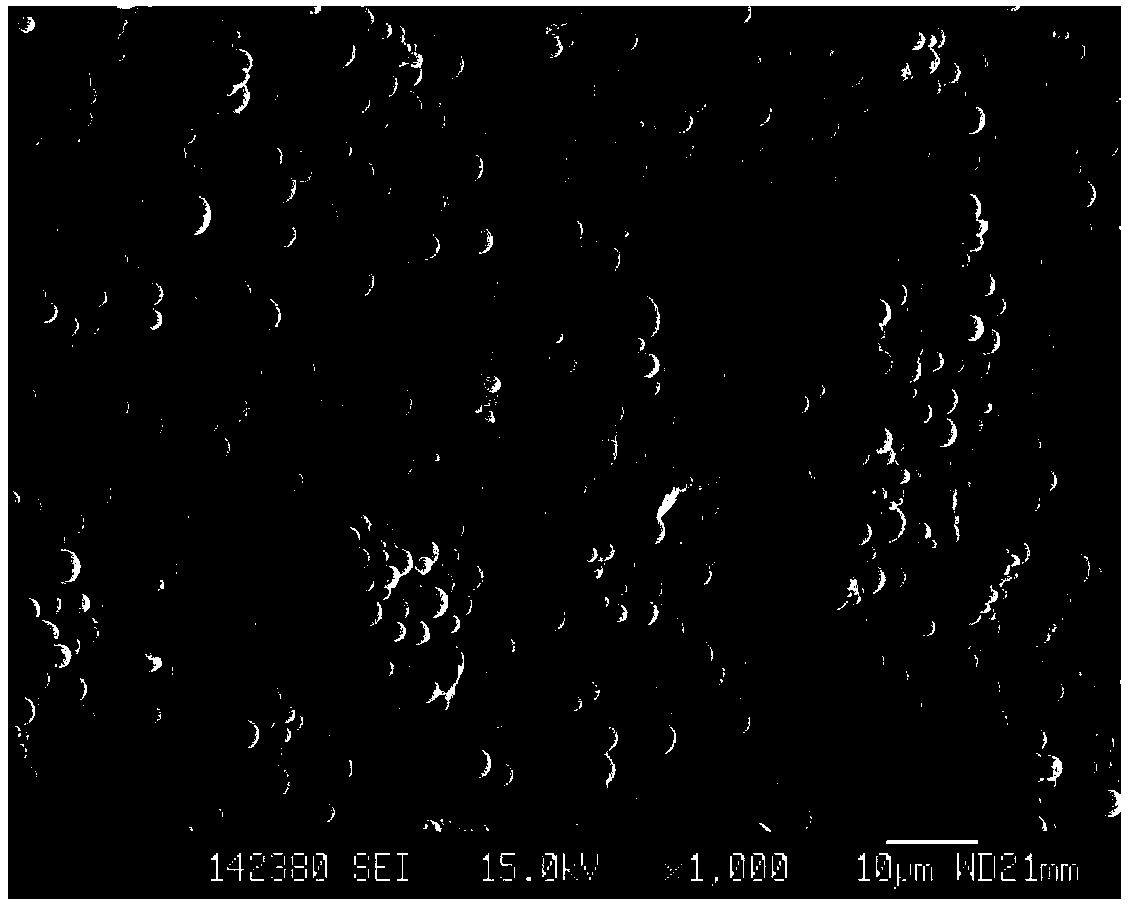
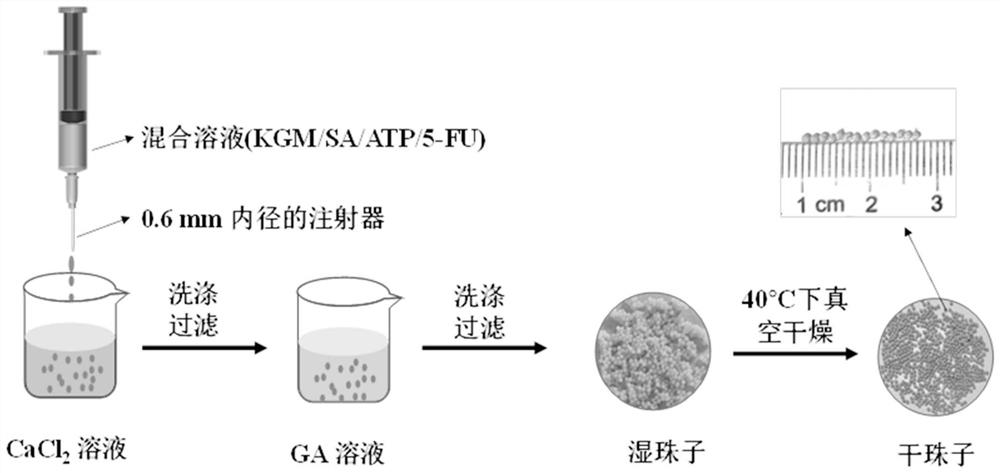
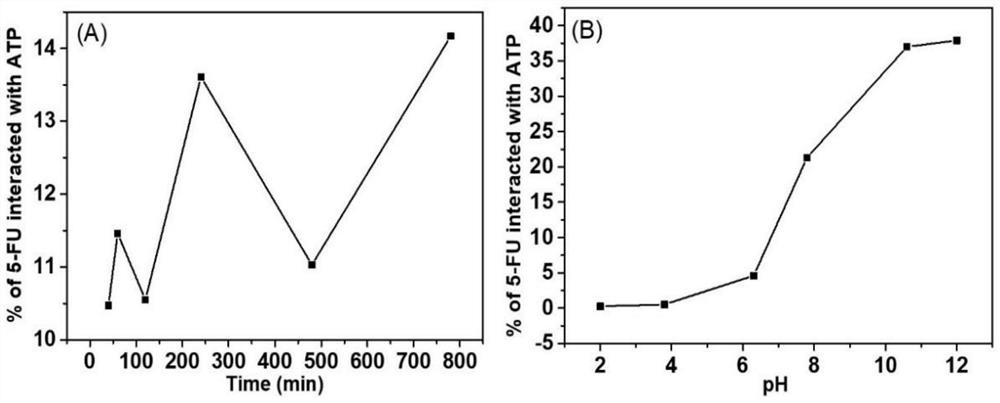
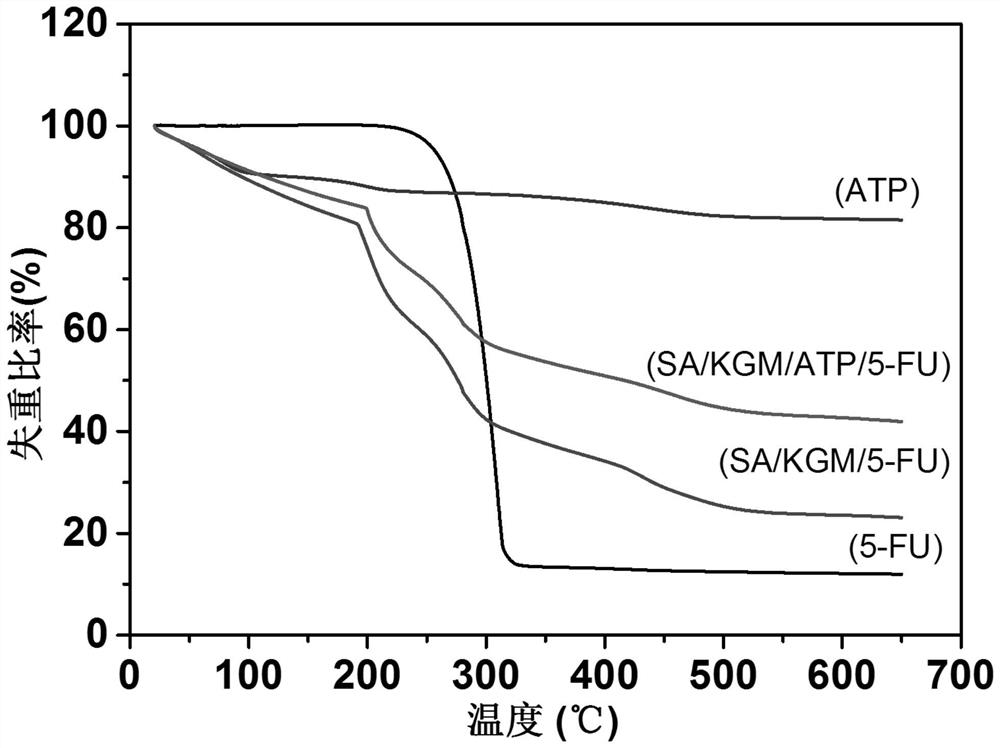
Konjac glucomannan sodium alginate composite drug-loaded microsphere as well as preparation method and application thereof
ActiveCN114668728AGood slow releaseLaiyuan richOrganic active ingredientsInorganic non-active ingredientsMicrosphereDrug encapsulation
The invention provides a konjac glucomannan and sodium alginate composite drug-loaded microsphere and a preparation method and application thereof.The preparation method of the composite drug-loaded microsphere comprises the following steps that a konjac glucomannan solution and a sodium alginate solution are mixed, attapulgite is added and stirred, fluorouracil is added and stirred, and the konjac glucomannan and sodium alginate composite drug-loaded microsphere is obtained. And injecting the solution into a CaCl2 solution by using an injector to form microspheres, transferring the microspheres into a glutaraldehyde solution, reacting, washing and drying. The inorganic mineral attapulgite is added into a natural high polymer material to increase the encapsulation and controlled release of the natural high polymer material to drugs, the composite material is prepared by a simple gel method, the encapsulation efficiency of fluorouracil is high, and the burst release phenomenon of 5-FU in a simulated solution can be obviously reduced; the preparation method disclosed by the invention is simple, the raw materials are rich, the thermal stability is good, the biocompatibility is good, the drug encapsulation efficiency is high, and the prepared composite drug-loaded microspheres are obvious in slow-release effect.
Owner:HUBEI UNIV FOR NATITIES
A kind of composite drug-carrying gel and preparation method thereof
ActiveCN113181108BImprove hydrophilicityGood water dispersibilityOrganic active ingredientsAerosol deliveryPolypyrroleDrug release
The invention provides a composite drug-carrying gel and a preparation method thereof. The preparation method includes the following steps: S1. Add pyrrole and dopamine hydrochloride into water and mix evenly, then add ammonium persulfate, stir and react, and centrifuge and wash to obtain polypyrrole -Polydopamine nanocomposite material; S2, placing the nanocomposite material in a 1mol / L hydrochloric acid solution containing drug molecules and a graphene oxide aqueous solution in turn, and centrifugal adsorption to obtain graphene oxide-polypyrrole-polydopamine nanocomposite drug-loading material; S3. After mixing the nano-composite drug-carrying material with the water-soluble polymer solution, adding calcium chloride solution, stirring, and letting it stand, the composite drug-carrying gel is obtained. The composite drug-carrying material provided by the invention is compounded with a water-soluble polymer, which can promote the formation of the composite drug-carrying gel, slow down the sudden release phenomenon at the initial stage of drug release, and at the same time, the composite drug-carrying gel has multiple stimuli responsiveness.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Topical pharmaceutical composition containing water-insoluble analgesic and preparation method and application thereof
InactiveCN110478366AIntegrity guaranteedAvoid sudden releaseHydroxy compound active ingredientsAntipyreticDrug release rateWater insoluble
The invention discloses a topical pharmaceutical composition containing a water-insoluble analgesic and a preparation method and application thereof. The topical composition includes a water-insolubleanalgesic drug and ozone. The water-insoluble analgesic drug is flurbiprofen axetil or propofol. The weight ratio of the water-insoluble analgesic drug to ozone is (10-100): (6-15). The drug encapsulation rate is improved and can reach 90% and above, which effectively reduces burst release of the drug. The drug release rate is stable and durable, and the sustained release period can reach 2 to 3days. The pharmaceutical composition is easy to apply and convenient to carry, and patient compliance is improved. The preparation method is simple, the conditions are mild, the preparation process issimple, parameters are controllable, reproducibility is good, production efficiency is high, and continuous large-scale production can be achieved. The pharmaceutical composition has low requirementson storage conditions and is easy to package, transport and store.
Owner:YICHANG HUMANWELL PHARMA
Injectable long-acting local anesthetic semi-solid formulations
ActiveCN113262302AApparent burst phenomenonReduce burst phenomenonOrganic active ingredientsSenses disorderLoteprednolGel preparation
The invention discloses an injectable long-acting semi-solid gel preparation which comprises a mixture containing ricinoleic acid triglyceride and a gelling agent and an active ingredient, and the active ingredient comprises loteprednol, latanoprost, celecoxib, triamcinolone acetonide or betamethasone. The pharmaceutical composition provided by the invention is a biologically erodible semisolid carrier gel preparation, has the advantage of sustained release of drugs, and does not have an obvious burst release phenomenon.
Owner:HUZHOU HUI ZHONG JI SHI BIOTECHNOLOGY CO LTD
Protein polypeptide drug long-acting microspheres and preparation method thereof
ActiveCN108403643BEasy to prepareMild preparation methodPeptide/protein ingredientsPharmaceutical non-active ingredientsMicrosphereWater soluble drug
The invention relates to protein polypeptide drug long-acting microspheres and a preparation method thereof. The preparation method comprises the steps of (1) dissolving a protein or polypeptide water-soluble drug and a protein protectant to obtain an aqueous drug solution; dissolving a macromolecular carrier material in an organic solvent to obtain an oil phase macromolecular solution; (2) addingthe aqueous drug solution into the oil phase macromolecular solution, and using a cell disrupter or a high-speed shear apparatus to prepare W / O emulsion; (3) feeding the W / O emulsion into a superfineparticle preparation system through a peristaltic pump so as to obtain the protein polypeptide drug long-acting microspheres. The protein polypeptide drug long-acting microspheres prepared via the preparation method have the advantages of good preparation process simplicity, controllable parameters, good reproducibility, and high production efficiency. The protein polypeptide drug long-acting microspheres prepared herein have the advantages of high drug encapsulating rate, low sudden release agent, stable and lasting release speed of drug, smooth microsphere surface, good spherical shape integrity, and homogenous particle size.
Owner:SUN YAT SEN UNIV
Recombined human vascellum esoderma inhibin durative action preparation, preparation method and application thereof
ActiveCN101292951BRelieve painReduce the burden onPeptide/protein ingredientsPharmaceutical delivery mechanismMicrosphereRelease time
The invention relates to the field of medical technology and discloses a long-acting preparation of lactic-glycolic acid copolymer (PLGA) for the recombination of endostatin of vein and the application to preparing a drug for treating tumor. The invention uses four methods of implanting a pump in vivo, implanting a microsphere in vivo, a W / O / W solvent volatilization method and a W / O / O solvent volatilization method to prepare the long-acting preparation. The sustained-release time of the long-acting preparation prepared is 7 to 28 days in accordance with the needs.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
A kind of microcapsules for encapsulation of small molecule hydrophilic drug sustained release and preparation method thereof
ActiveCN104224753BHigh viscosityReduce spreadPharmaceutical delivery mechanismMacromolecular non-active ingredientsEmbedding rateSustained Release Capsule
The invention relates to a method for preparing a small molecule hydrophilic drug-embedded sustained-release capsule. The method comprises the following steps: filtering a prepared'internal aqueous phase / oil phase / external aqueous phase' pre-multi-emulsion solution through a microporous membrane; and then removing a solvent, washing and drying to obtain the small molecule hydrophilic drug-embedded sustained-release capsule, wherein the internal aqueous phase comprises a small molecule hydrophilic drug and a thickening agent. The small molecule hydrophilic drug-embedded sustained-release capsule provided by the invention has the advantages of uniform grain diameter, high embedding rate, low burst-release rate, stable drug release and long action.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for preparing surface-closed medicine-carrying porous polymer microsphere based on supercritical fluid technology
InactiveCN102552167BResidue reductionActive completeOrganic active ingredientsPharmaceutical product form changeWater bathsMicrosphere
The invention discloses a method for preparing surface-closed medicine-carrying porous polymer microsphere based on a supercritical fluid technology. The method comprises the following steps of: dispersing a dichloromethane solution of a polymer, poloxamer serving as a pore-foaming agent and a medicinal mixture in a polyvinyl alcohol aqueous solution to form emulsion, performing water bath and drying to obtain a medicine-carrying communication porous polymer microsphere; putting the medicine-carrying communication porous polymer microsphere into a high-pressure autoclave; and introducing supercritical carbon dioxide to contact the medicine-carrying communication porous polymer microsphere fully, closing communication holes on the surface of polymer microsphere under the plasticizing action of the supercritical carbon dioxide on a polymer, and releasing pressure to form the surface-closed medicine-carrying porous polymer microsphere. The method has a simple operating process, is mild in operating conditions and stable in process; and by the method, solvent residues can be removed in the process for preparing the medicine-carrying communication porous polymer microsphere, the burst release of medicines is avoided, and the method can be applied to sustained and controlled release administration systems.
Owner:ZHEJIANG UNIV
Rebamipide sustained release tablet and preparation method thereof
PendingCN114404379AReduced bioavailabilityReduce solubilityOrganic active ingredientsDigestive systemCarboxymethyl celluloseProlonged-release tablet
The invention relates to the technical field of pharmaceutical preparations, in particular to a rebamipide sustained release tablet and a preparation method thereof. The rebamipide sustained release tablet is prepared from rebamipide, a framework material and a microcrystalline cellulose-carboxymethyl cellulose sodium compound. The preparation method comprises the following steps: firstly, mixing the rebamipide with the framework material and the microcrystalline cellulose-carboxymethylcellulose sodium compound, and granulating; and mixing the granulated particles with a lubricant, tabletting, and coating. The rebamipide sustained release tablet provided by the invention not only can improve the bioavailability of the rebamipide tablet, but also can improve the common burst release phenomenon of a sustained release preparation, not only has important clinical significance, but also can reduce the production cost.
Owner:杭州沐源生物医药科技有限公司
Water-soluble drug sustained-release microsphere and preparation method and applications thereof
InactiveCN101444486BHigh encapsulation efficiencyLeak blockOrganic active ingredientsPeptide/protein ingredientsDispersed mediaOrganic solvent
Owner:GUANGDONG PHARMA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com