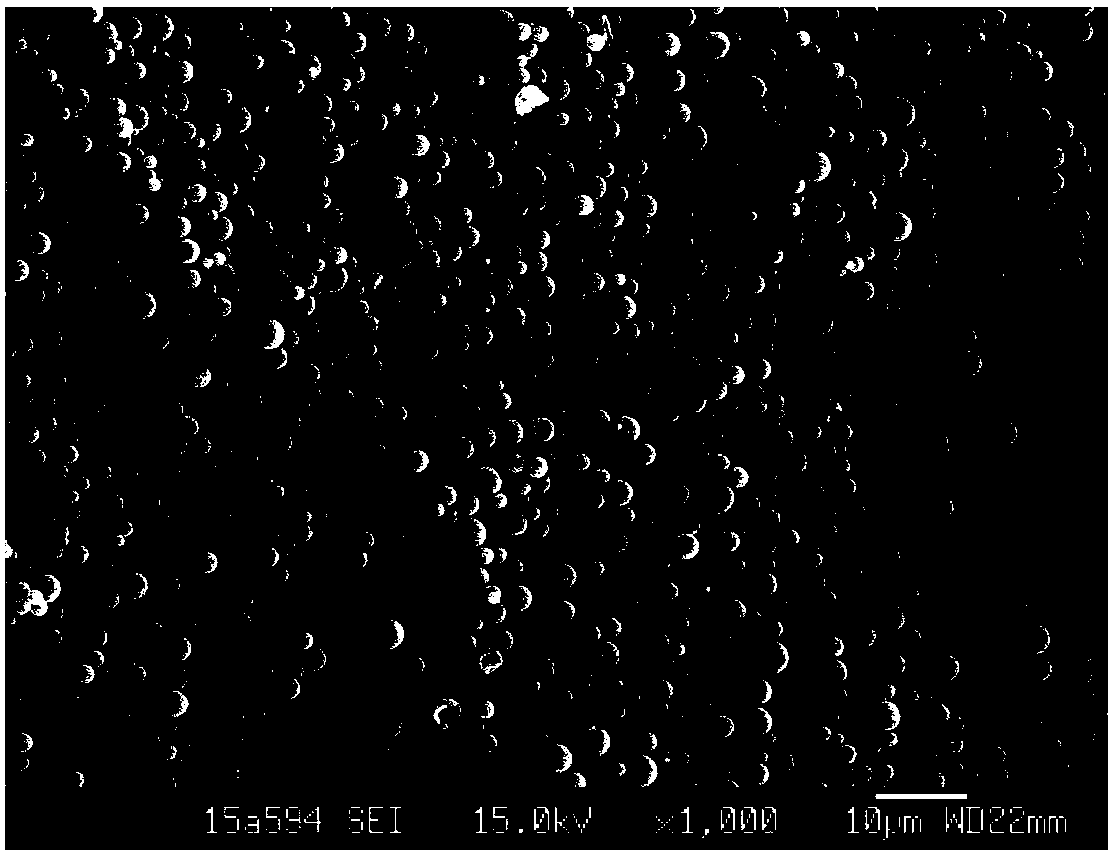
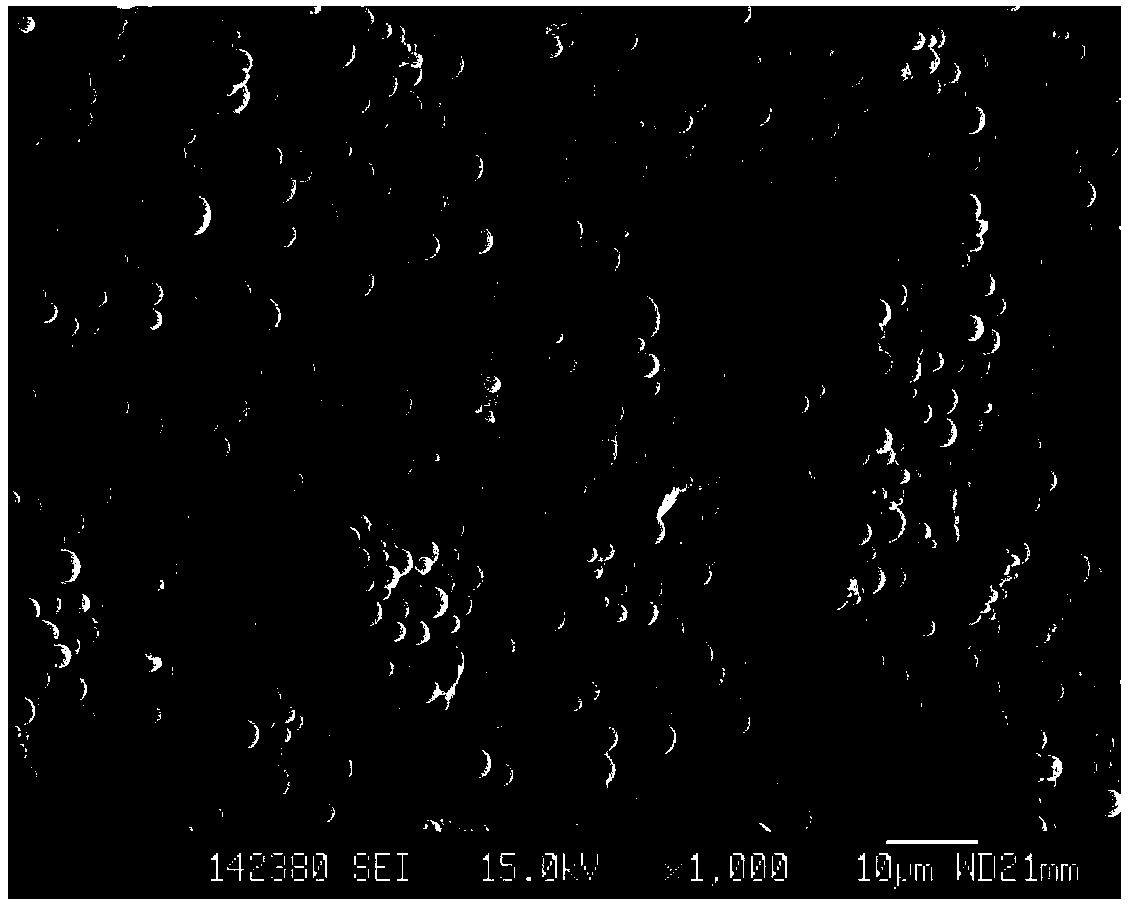
Phospholipid protein particle composite microsphere and preparation method thereof
A technology of protein particles and composite microspheres, applied in peptide/protein components, microcapsules, pharmaceutical formulations, etc., can solve the problems of protein drug activity decline, protein drug aggregation, burst release, etc., to achieve low burst release rate, drug package High sealing rate and solving the effect of high burst release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Preparation of phospholipid-protein microparticle composite microspheres of bovine serum albumin
[0039] The preparation method of the bovine serum albumin phospholipid protein particle composite microsphere of the present embodiment comprises the following steps:
[0040] 1. Preparation of phospholipid protein microparticles
[0041] Weigh an appropriate amount of water-soluble model protein drug bovine serum albumin and freeze-drying protective agent trehalose and dissolve them in ultrapure water to prepare a solution with a concentration of bovine serum albumin of 2 mg / mL and a concentration of trehalose of 1 mg / mL as the aqueous phase , Weigh an appropriate amount of soybean phosphatidylcholine and dissolve it in tert-butanol to prepare a tert-butanol solution with a phospholipid concentration of 50 mg / mL as the oil phase. Under the conditions of a water bath of 37° C. and a magnetic stirring rate of 1300 rpm, the oil phase was added dropwise to the ...
Embodiment 2
[0050] Embodiment 2: the preparation of the phospholipid protein particle composite microsphere of bovine serum albumin
[0051] The preparation method of the bovine serum albumin phospholipid protein particle composite microsphere of the present embodiment comprises the following steps:
[0052] 1. Preparation of phospholipid protein microparticles
[0053] Weigh an appropriate amount of water-soluble model protein drug bovine serum albumin and freeze-drying protective agent mannitol and dissolve them in ultrapure water to prepare a solution with a concentration of bovine serum albumin of 2 mg / mL and a concentration of mannitol of 1 mg / mL as the aqueous phase , Weigh an appropriate amount of soybean phosphatidylcholine and dissolve it in tert-butanol to prepare a tert-butanol solution with a phospholipid concentration of 50 mg / mL as the oil phase. Under the conditions of a water bath of 37° C. and a magnetic stirring rate of 1300 rpm, the oil phase was added dropwise to the ...
Embodiment 3
[0060] Example 3: Preparation of Thymopentin Phospholipid Protein Microparticle Composite Microspheres
[0061] The preparation method of the phospholipid protein particle composite microsphere of Thymopentin of the present embodiment comprises the steps:
[0062] 1. Preparation of phospholipid protein microparticles
[0063] Weigh an appropriate amount of water-soluble oligopeptide drug thymopentin and freeze-drying protective agent trehalose and dissolve them in ultrapure water to prepare a solution with a thymopentin concentration of 2 mg / mL and a trehalose concentration of 1 mg / mL as the aqueous phase, weighing An appropriate amount of soybean phosphatidylcholine was dissolved in tert-butanol to prepare a tert-butanol solution with a phospholipid concentration of 50 mg / mL as the oil phase. Under the conditions of a water bath of 37° C. and a magnetic stirring rate of 1300 rpm, the oil phase was added dropwise to the water phase, and the volume ratio of the oil phase to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com