Drug-loaded liposome and preparation method thereof
A technology of liposomes and blank liposomes, which is applied in liposome delivery, pharmaceutical formulations, drug combinations, etc., can solve problems affecting the effectiveness, safety and stability of preparations, poor thermal stability, drug leakage, etc., and achieve improved Effects of drug encapsulation efficiency, inhibition of flocculation, and enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
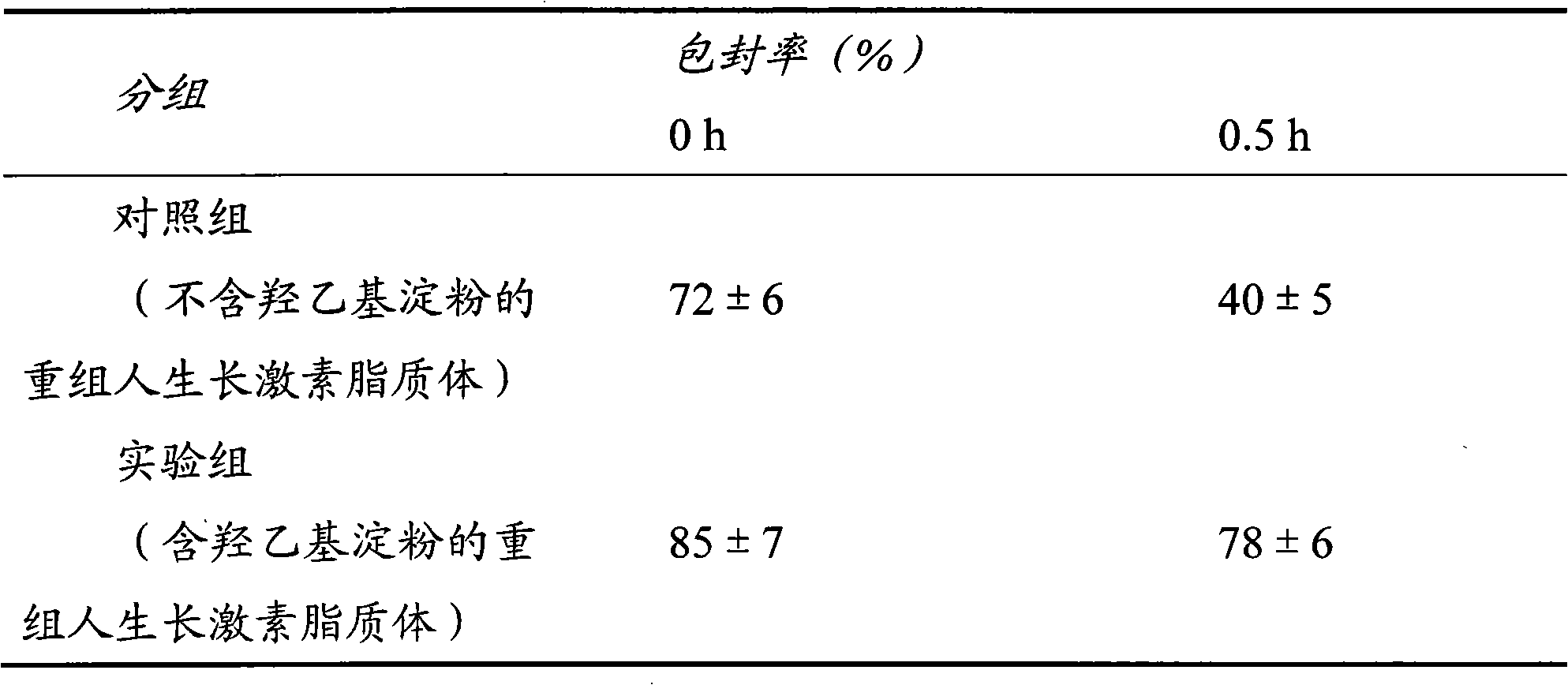
[0034] Embodiment 1: recombinant human growth hormone liposome
[0035] Although many biotechnology drugs are highly active, they have poor stability and need to be entrapped in microparticles to improve their stability and prolong drug release. The first embodiment of the present invention uses recombinant human growth hormone as a target drug, uses hydrogenated egg yolk phospholipid and medium molecular weight hydroxyethyl starch as materials, and adopts a passive drug loading method to prepare recombinant human growth hormone liposome.
[0036] Preparation of blank liposomes: Using the ethanol injection method, weigh 48 mg of hydrogenated egg yolk phospholipids and 16 mg of cholesterol, add them to 20 ml of absolute ethanol, and sonicate until completely dissolved to form an organic phase. Weigh 10 mg of Tween 80 and add it into 20 ml of 0.02 mol / L phosphate buffer saline (PBS) with pH=7, and mix well to form the aqueous phase. Inject the organic phase into the water phase...
Embodiment 2
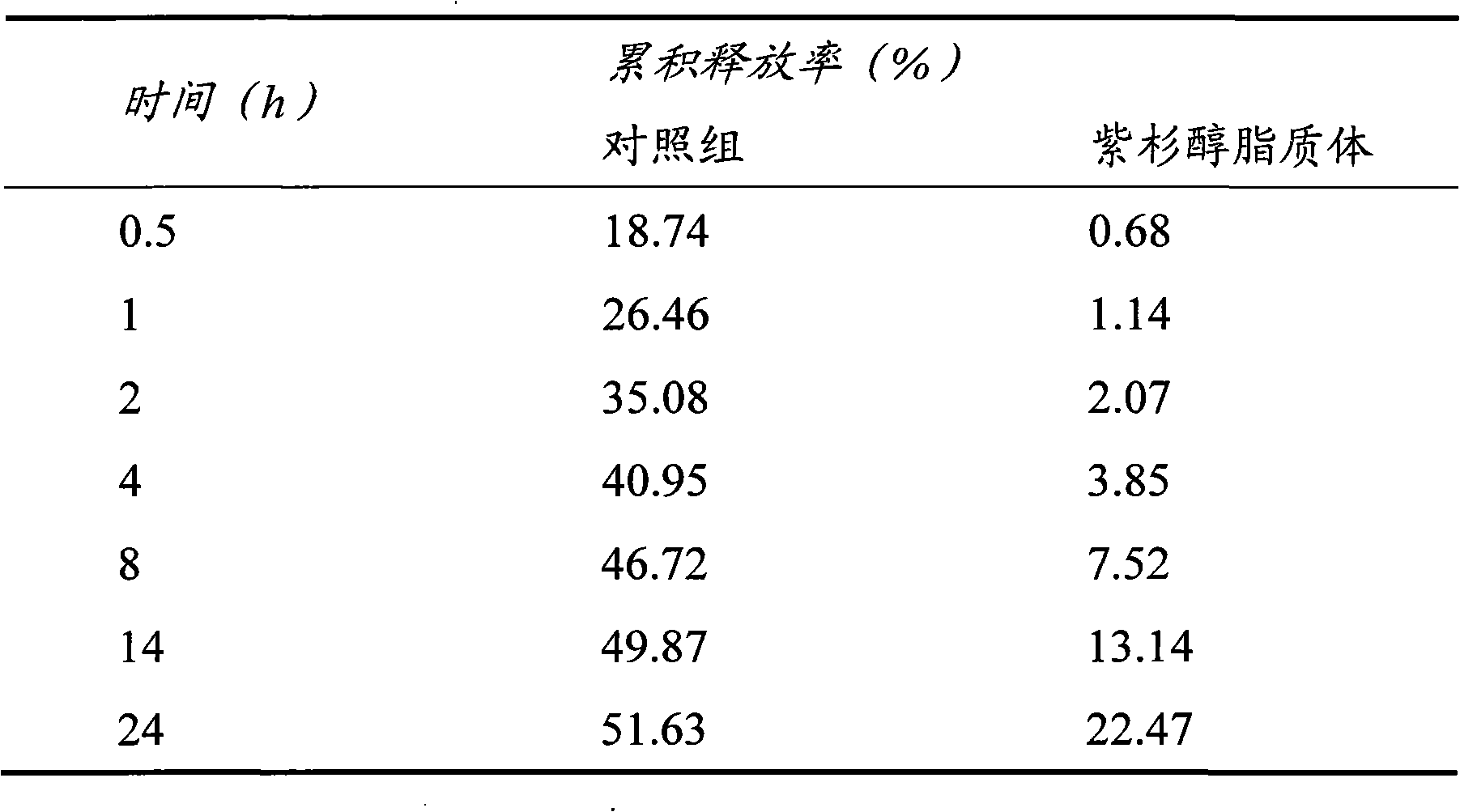
[0045] Embodiment 2: Paclitaxel liposome
[0046] Some drugs need to be long-acting to reduce the number of doses. In the second embodiment of the present invention, the antineoplastic drug paclitaxel is used as the target drug, and the paclitaxel liposome is prepared by using synthetic phospholipids and high-molecular weight and high-substituted hydroxyethyl starch as materials.
[0047] Preparation of paclitaxel liposomes: 5mg paclitaxel, 6mg dipalmitoylphosphatidylcholine (DPPC), 1mg polyethylene glycol 2000 grafted distearoylphosphatidylethanolamine (DSPE-PEG2000) added 16ml chloroform: methanol (3 : 1, V / V) is dissolved in a mixed solvent to form an organic phase. Weigh 10 mg of Tween 80 and add it into 20 ml of 0.02 mol / L phosphate buffer saline (PBS) with pH=7, and mix well to form the water phase. Inject the organic phase into the water phase at (25±2)°C stirred at 1000r / min, stir at 1000r / min for 10min to form a liposome suspension, remove the organic solvent by rot...
Embodiment 3
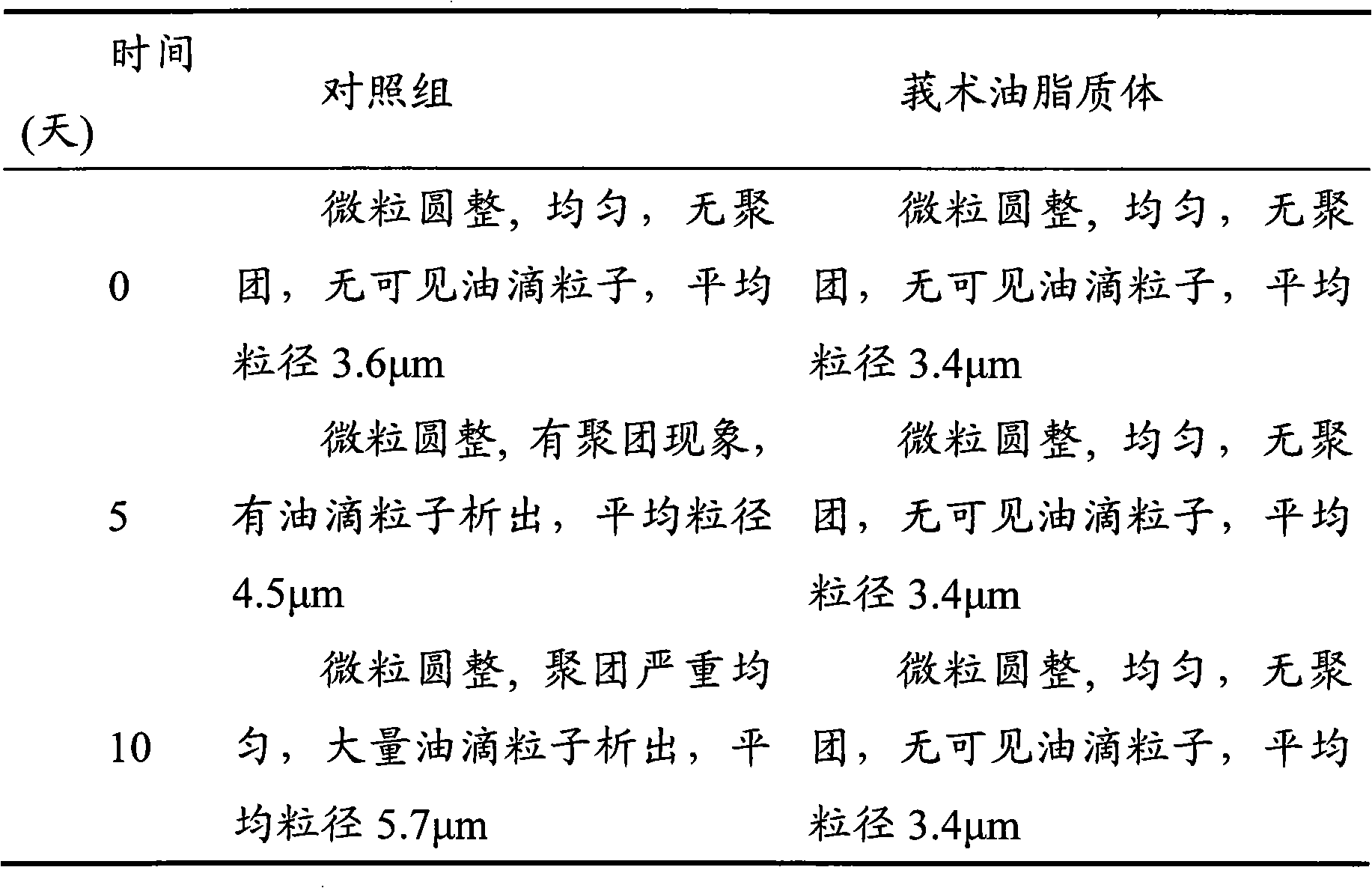
[0053] Embodiment 3: Curcuma oil liposome
[0054] Many liquid insoluble drugs such as plant volatile oil are not suitable for making various preparations directly. In the third embodiment of the present invention, the liquid insoluble drug zedoary oil is used as the target drug, and hydrogenated soybean lecithin and low-molecular-weight hydroxyethyl starch are used to prepare nano-scale zedoary oil liposomes, which are convenient for making various preparations.
[0055] Preparation of zedoary oil liposomes: Take 0.5g Tween 80, 1g zedoary oil and 0.5g hydrogenated soybean lecithin, add 10ml ethanol, dissolve in a water bath at 70°C, and form an organic phase. Weigh 1g of gelatin and 1g of hydroxyethyl starch 40 and dissolve in 40ml of distilled water at 70°C as the water phase. Under the condition of stirring at 3000r / min, slowly inject the oil phase into the water phase with a preheated syringe, and stir at a high speed for 1 hour to obtain a zedoary oil liposome suspension...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com