Rebamipide sustained release tablet and preparation method thereof
A technology of rebamipide and sustained-release tablets, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients. and other issues, to achieve good compliance, reduce toxicity, and reduce the amount of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
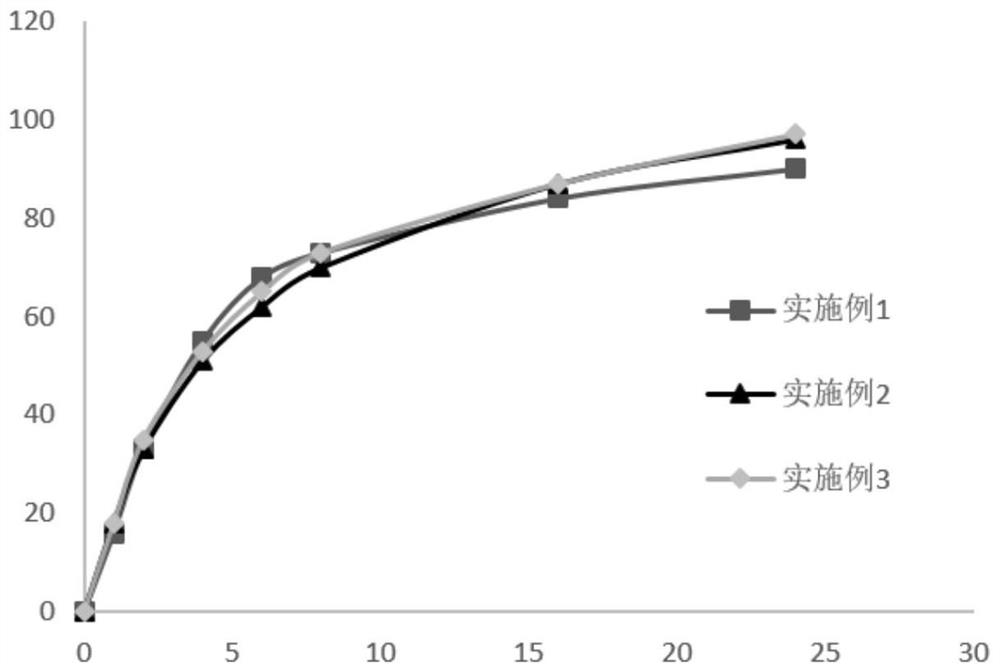
Embodiment 1
[0042] The formulation of the rebamipide sustained-release tablet in this embodiment is shown in Table 1 below.
[0043] Table 1 Recipe table
[0044]
[0045]
[0046] The preparation method is as follows:
[0047] (1) After mixing the raw materials except the lubricant magnesium stearate, wet granulate with 70% ethanol solvent;
[0048] (2) drying wet granulated granules, and then mixing with a lubricant for tableting; mixing hypromellose and titanium dioxide with a mass ratio of 1:1 to obtain a film coating premix;
[0049] (3) Dissolving the film coating premix with ethanol to obtain a coating solution, and coating the tablet with the coating solution.
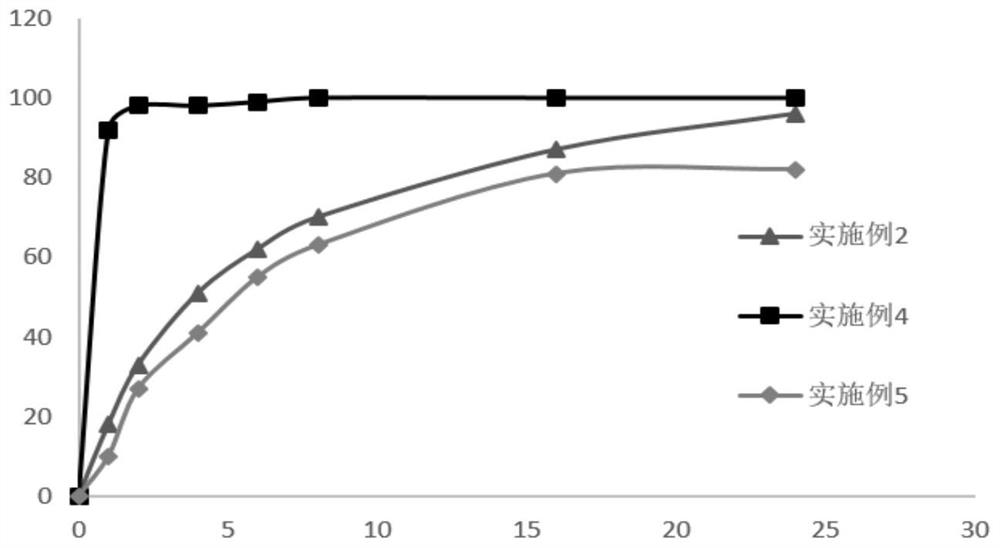
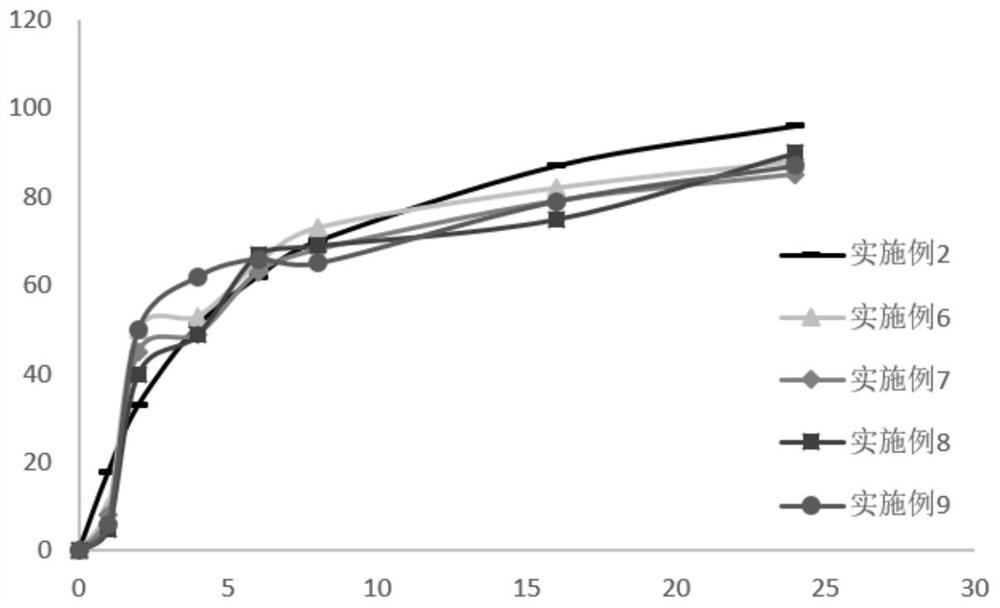
Embodiment 2
[0051] The formulation of the rebamipide sustained-release tablet in this embodiment is shown in Table 2.
[0052] Table 2 Formulation table
[0053]
[0054] The preparation method is as follows:
[0055] (1) After mixing the raw materials except lubricant, wet granulation with 70% ethanol solvent;
[0056] (2) The dried wet granulated granules are mixed with a lubricant and pressed into tablets; polyvinyl alcohol, lecithin and titanium dioxide are mixed with a mass ratio of 1:0.5:1 to obtain a film coating premix;
[0057](3) Dissolving the film coating premix with ethanol to obtain a coating solution, and coating the tablet with the coating solution.
Embodiment 3
[0059] The formulation of the rebamipide sustained-release tablet in this embodiment is shown in Table 3 below.
[0060] Table 3 recipe table
[0061]
[0062]
[0063] The preparation method is as follows:
[0064] (1) After mixing the raw materials except lubricant, wet granulation with 70% ethanol solvent;
[0065] (2) The dried wet granulated granules are then mixed with a lubricant and compressed into tablets;
[0066] (3) Dissolving the film coating premix with ethanol to obtain a coating solution, and coating the tablet with the coating solution. Wherein the film coating premix is hypromellose.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com