Microsphere for double protection of antibody drug and intravitreal injection, and preparation method thereof
A double-protection, anti-antibody technology, applied in the field of medicine, can solve problems such as the reduction of drug loading and encapsulation efficiency, antibody denaturation and aggregation, and large molecular weight of drugs, so as to improve denaturation and aggregation, reduce irritation, and improve patient compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
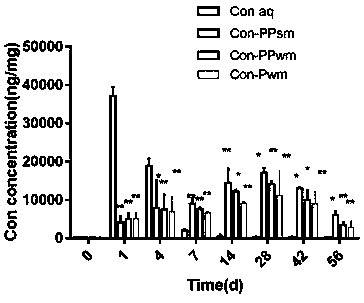
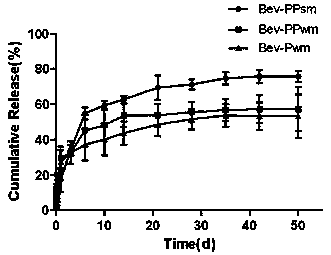
[0029] Note: Bev-PPsm in the experimental group is PLGA / PCADK microspheres loaded with bevacizumab prepared by S / O / W method; Bev-PPwm in the control group is loaded with bevacizumab prepared by W / O / W PLGA / PCADK microspheres of vacizumab; the control group Bev-Pwm is PLGA microspheres loaded with bevacizumab prepared by W / O / W method;
[0030] Bev-PPsm Prescription
[0031] Phase S: bevacizumab-dextran microparticles (1:3), 50mg;
[0032] Phase O: PLGA 7525 4A, 80 mg; PCADK, 20 mg; Dichloromethane, 2 mL;
[0033] Phase W: 1.0% PVA aqueous solution, 10 mL;
[0034] Preparation process of Bev-PPsm
[0035] 1) Dissolve bevacizumab, dextran and PEG (1: 3: 40) in deionized water, vortex and then freeze-dry; the powder obtained after freeze-drying is washed by centrifugation with dichloromethane to remove the PEG dispersed phase, Vacuum drying to obtain bevacizumab-dextran microparticles;
[0036] 2) Dissolve PLGA and PCADK in dichloromethane, add the prepared particles, and ul...
Embodiment 2
[0050] Note: Afl-PPsm in the experimental group is the PLGA / PCADK microspheres loaded with aflibercept prepared by the S / O / W method; Afl-PPwm in the control group is prepared by the W / O / W method PLGA / PCADK microspheres of Aflibercept; the control group Afl-Pwm is PLGA microspheres loaded with Aflibercept prepared by W / O / W method;
[0051] Afl-PPsm prescription
[0052] Phase S: Aflibercept-dextran microparticles (1:4), 40mg;
[0053] Phase O: PLGA 7525 4A, 70mg; PCADK, 30mg; Dichloromethane, 2 mL;
[0054] Phase W: 2.0% PVA aqueous solution, 15 mL;
[0055] Preparation process of Afl-PPsm
[0056] 1) Dissolve aflibercept, dextran and PEG (1:4:40) in deionized water, vortex and then freeze-dry; the powder obtained after freeze-drying is washed by centrifugation with dichloromethane to remove the PEG dispersed phase, Vacuum drying to obtain aflibercept-dextran microparticles;
[0057] 2) Dissolve PLGA and PCADK in dichloromethane, add the prepared particles, and ultrasonica...
Embodiment 3
[0071] Note: The experimental group Luc-PPsm is the PLGA / PCADK microspheres loaded with ranibizumab prepared by the S / O / W method; the control group Luc-PPwm is the ranibizumab-loaded microspheres prepared by the W / O / W method PLGA / PCADK microspheres of zizumab; the control group Luc-Pwm is PLGA microspheres loaded with ranibizumab prepared by W / O / W method;
[0072] Luc-PPsm Prescription
[0073] Phase S: ranibizumab-dextran microparticles (1:5), 48mg;
[0074] Phase O: PLGA 7525 5A, 80mg; PCADK, 20mg; Dichloromethane, 2 mL;
[0075] Phase W: 1.0% PVA aqueous solution, 20 mL;
[0076] Preparation process of Luc-PPsm
[0077] 1) Weigh ranibizumab, dextran and PEG (1:5:40) and dissolve in deionized water, vortex and then freeze-dry; the powder obtained after freeze-drying is centrifuged with dichloromethane to remove the PEG dispersed phase, Vacuum drying to obtain ranibizumab-dextran microparticles;
[0078] 2) Dissolve PLGA and PCADK in dichloromethane, add the prepared p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com