Konjac glucomannan sodium alginate composite drug-loaded microsphere as well as preparation method and application thereof
A technology of konjac glucomannan and sodium alginate, which is applied in the direction of pharmaceutical formulations, drug combinations, anti-tumor drugs, etc., to achieve the effect of rich raw materials, good thermal stability, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
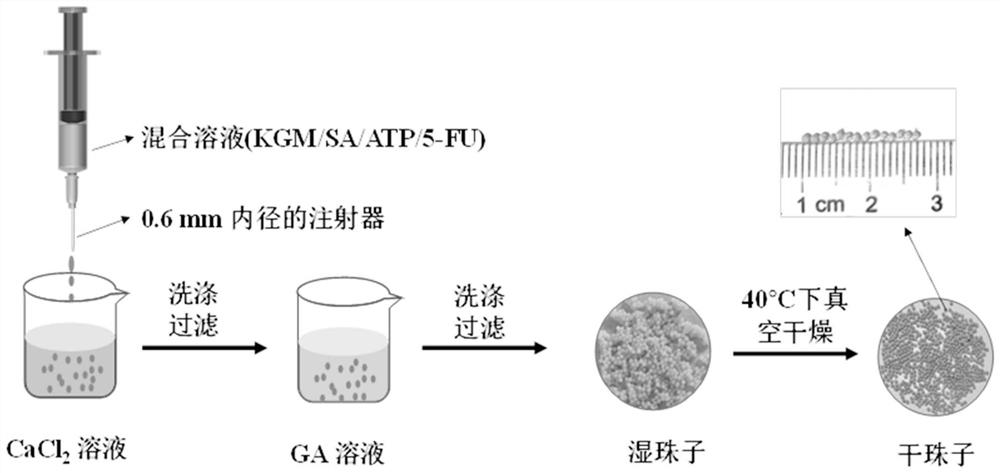
[0032] The embodiment of the present application provides a preparation method of konjac glucomannan sodium alginate composite drug-carrying microspheres, comprising the following steps:
[0033] S1, after mixing konjac glucomannan solution and sodium alginate solution, obtain the first mixed solution;
[0034] S2, adding attapulgite to the first mixed solution, stirring evenly, to obtain a second mixed solution;
[0035] S3. Add fluorouracil to the second mixed solution, stir, and then inject the solution into CaCl using a syringe 2 In the solution, microspheres are formed, and then the microspheres are transferred into a glutaraldehyde solution for cross-linking reaction, washed and dried to obtain konjac glucomannan and sodium alginate composite drug-carrying microspheres.
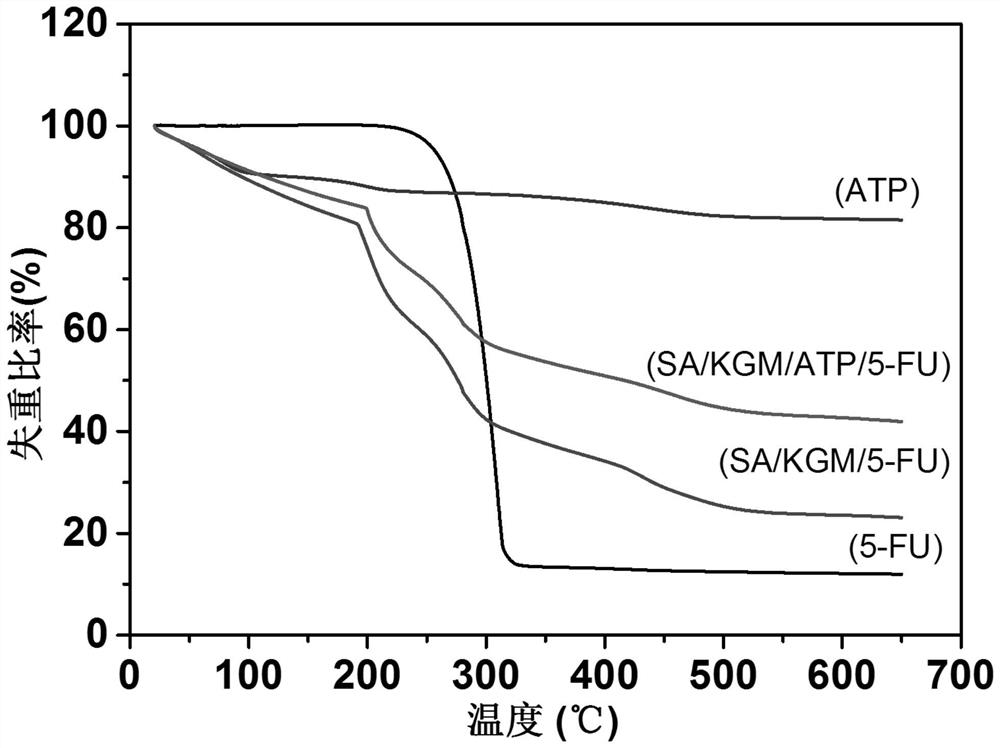
[0036] The preparation method of konjac glucomannan sodium alginate composite drug-carrying microspheres of the present invention adopts the matrix materials all from natural products, and the inorgani...
Embodiment 1
[0065] The embodiment of the present application provides a preparation method of konjac glucomannan sodium alginate composite drug-carrying microspheres, comprising the following steps:
[0066] S1, after mixing the konjac glucomannan solution with a mass concentration of 1% and a sodium alginate solution with a mass concentration of 3%, use a magnetic stirrer to stir for 2h to obtain a first mixed solution; wherein, the konjac glucomannan solution and the seaweed The volume ratio of the sodium solution is 1:9;
[0067] S2, adding 400mg attapulgite to the first mixed solution, stirring evenly, to obtain the second mixed solution;
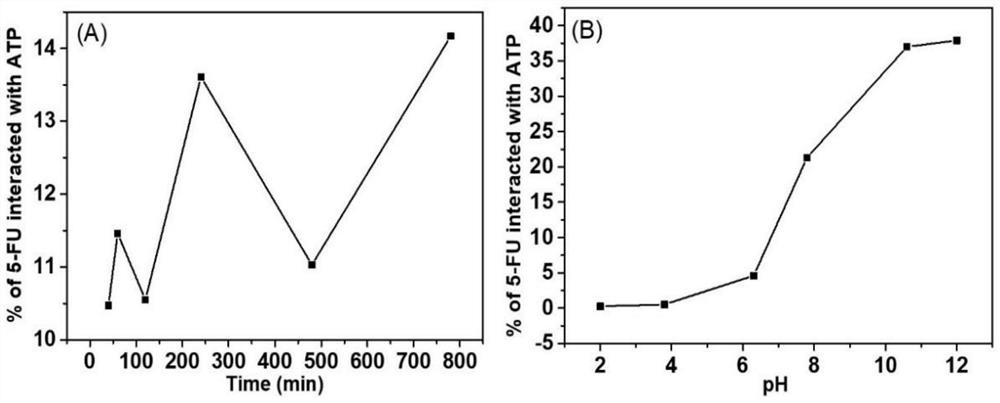
[0068] S3. Adjust the pH of the second mixed solution to 11 with 0.1 mol / L NaOH solution, stir evenly, then ultrasonicate for 30 minutes, then add 100 mg of fluorouracil and continue to stir for 60 minutes; then use a syringe with an inner diameter of 0.6 mm to make the solution Injected into CaCl with a mass concentration of 2% 2 In the solution...
Embodiment 2
[0074] The embodiment of the present application provides a preparation method of konjac glucomannan sodium alginate composite drug-carrying microspheres, comprising the following steps:
[0075] S1, after mixing the konjac glucomannan solution with a mass concentration of 1% and a sodium alginate solution with a mass concentration of 3%, use a magnetic stirrer to stir for 2h to obtain a first mixed solution; wherein, the konjac glucomannan solution and the seaweed The volume ratio of the sodium solution is 1:9;
[0076] S2, adding 200mg attapulgite to the first mixed solution, stirring evenly, to obtain the second mixed solution;
[0077] S3. Adjust the pH of the second mixed solution to 12 with 0.1mol / L NaOH solution, stir evenly, then ultrasonicate for 30 minutes, then add 150 mg of fluorouracil and continue to stir for 60 minutes; then use a syringe with an inner diameter of 0.6 mm to make the solution Injected into CaCl with a mass concentration of 2% 2 In the solution,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com