Polypeptide HM-3 nanometer particles and preparation method thereof
A nanoparticle, HM-3 technology, applied in pharmaceutical formulations, peptide/protein components, medical preparations with non-active ingredients, etc., can solve problems such as toxic side effects, blood drug concentrations approaching or exceeding poisoning levels, etc., and achieve the preparation method. Simple, increase the encapsulation efficiency and drug loading, and prolong the half-life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
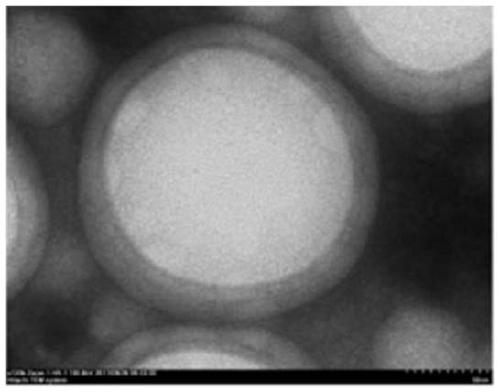
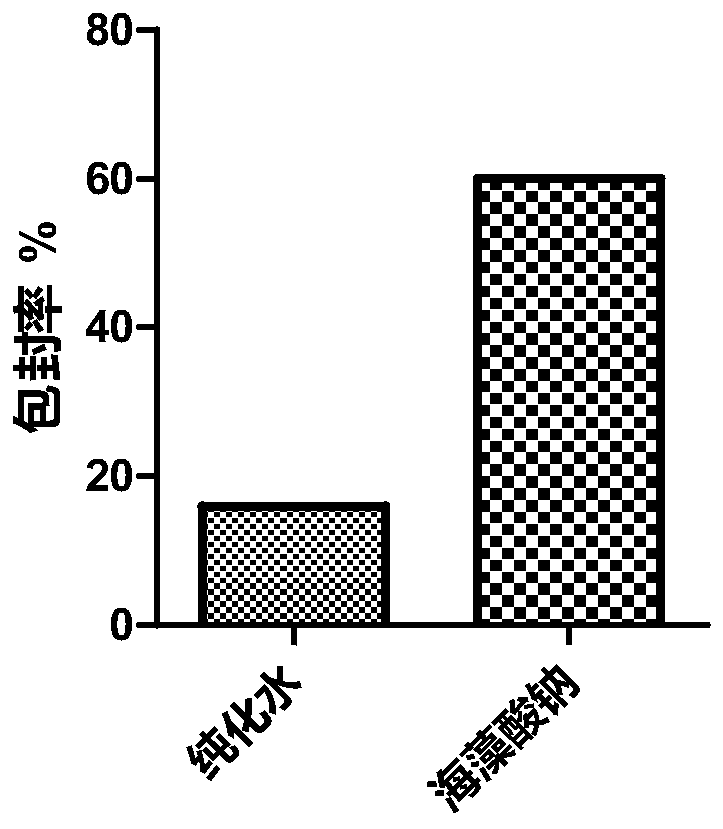
[0037] Example 1: Weigh about 100mg of PLGA (50:50), dissolve in 4ml of dichloromethane as the organic phase; take another 5mg of HM-3, dissolve in 200μL of 0.1% sodium alginate as the inner water phase, and mix the two phases Ultrasonic (150w, 1min) after the probe, fully emulsified and injected into 16ml mixed solution (continuing filtrate of 1% PVA, 4% chitosan solution + 5% calcium chloride mixed solution), stirred at room temperature to evaporate the organic solvent, and stirred at a low speed of 5000rpm 20min, washed 3 times with water, and freeze-dried. Obtain the PLGA nanoemulsion modified by sodium alginate and chitosan with uniform particle size and round shape, see figure 1 .
Embodiment 2
[0038] Embodiment 2: dissolution test
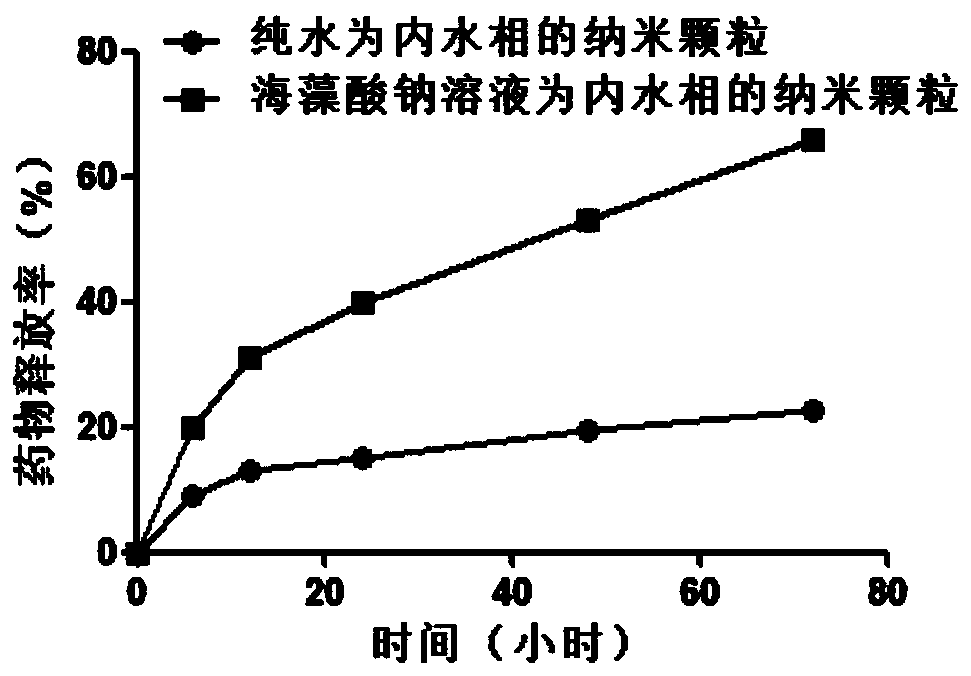
[0039] Two PLGA nanoparticles loaded with the same concentration of HM-3 were dissolved in saline and placed in an ultrafiltration tube, placed in a shaker (37°C, 75rpm), and centrifuged regularly (4°C, 7000rpm, 20min) to collect 1ml Ultrafiltrate, while supplementing an equal amount of release medium. Agilent high performance liquid chromatography was used to measure the content of HM-3 in the ultrafiltrate, and then the cumulative release rate at different time points was calculated. The experimental results are shown in image 3 .
Embodiment 3
[0040] Embodiment 3: in vivo anti-tumor test
[0041] Establishment of xenograft tumor model in black mice: Inoculate murine melanoma cells into the subcutaneous axillary of C57BL / 6 black mice, and the inoculation volume of each black mouse is 2×10 6 cells / 0.1mL, when the tumor grows to a certain size, dissect the black mouse to take out the tumor tissue, cut it into pieces and grind it, pass it through a cell sieve, absorb and centrifuge the filtered cell solution, and re-mix it in the blank medium to make the cells The concentration is 2×10 7 individual / mL. Begin to inoculate the cells in the skin of the right armpit of black mice, and the inoculation volume of each black mouse is 1×10 6 indivual. When the tumor tissue grows to 70-90mm 3 The diameter of the tumor tissue was measured with a vernier caliper. There were 16 mice in the negative control group and 10 mice in the treatment group. The black mice were selected to ensure uniform tumor volume and good growth statu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com