Water-soluble drug sustained-release microsphere and preparation method and applications thereof
A technology of water-soluble drugs and sustained-release microspheres, which is applied in the direction of drug combinations, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of oil film rupture, low drug encapsulation rate, and short release time in vitro, and achieve uniform particle size distribution, The preparation process is stable and the effect of reducing the burst release phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Model Drug Metformin Hydrochloride Sustained Release Microspheres and Its Preparation
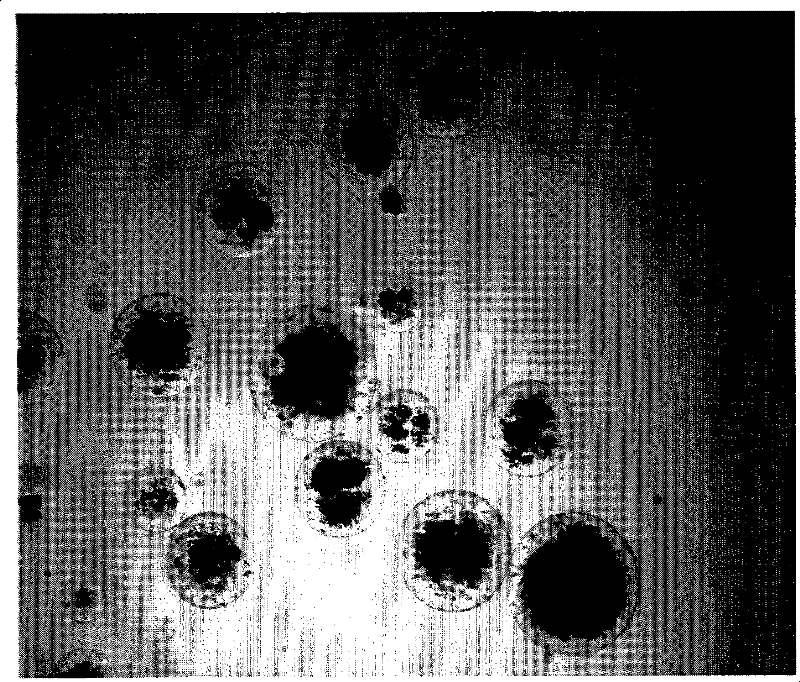
[0037] Metformin hydrochloride sustained-release microspheres are storage type, divided into two areas, the inner core area is composed of water-soluble drug and isolated oil phase, and the outer area is diffusion area, which is composed of polymer substances, and the isolated oil phase is in water-soluble drug phase. between polymers. The average particle size of the microspheres is 10-250 μm.
[0038] Its concrete preparation method is as follows, and the reagent that adopts is the preparation (following examples are the same) of pharmaceutical technical field routine use:
[0039] (1) dissolving pharmaceutical polymer excipients in an organic solvent to form a dispersion medium;
[0040] 7g Eudragit RS PO was dissolved in 50ml ethanol to prepare acrylic resin solution (O 2 ).
[0041] (2) Uniformly disperse the water-soluble drug powder in the oil phase of the isolat...
Embodiment 2
[0050] Example 2 Preparation of model drug insulin sustained-release microspheres
[0051] (1) dissolving pharmaceutical polymer excipients in an organic solvent to form a dispersion medium;
[0052] 200mg Eudragit RS PO and 200mg Eudragit RL PO were dissolved in 10ml ethanol to prepare acrylic resin solution (O 2 );
[0053] (2) Evenly disperse the water-soluble drug powder in the oil phase of the isolation layer to form a suspension (S / O 1 ) and slowly added to the dispersion medium, emulsified to make colostrum;
[0054] Disperse 50 mg of insulin powder evenly in 100 mg of peanut oil containing Span 804% (g / g);
[0055] The above-mentioned hydrophilic drug suspension (S / O 1 ) was slowly added to the acrylic resin solution (O 2 ), ultrasonication for 2min (power 40W) to make S / O 1 / O 2 type emulsion;
[0056] (3) re-emulsification of colostrum to obtain double milk, and volatilization of organic solvents to form microspheres;
[0057] The above S / O 1 / O 2 Colostru...
Embodiment 3
[0062] Example 3 Preparation of Model Drug Interferon (IFN-α) Sustained Release Microspheres
[0063] (1) dissolving pharmaceutical polymer excipients in an organic solvent to form a dispersion medium;
[0064] 50mgPLGA (LA:GA=50:50, specific viscosity is 0.19dl / g) was dissolved in 5ml acetonitrile to prepare PLGA solution (O 2 );
[0065] (2) Uniformly disperse the water-soluble drug powder in the oil phase of the isolation layer to form a suspension, slowly add it to the dispersion medium, and emulsify it to make colostrum;
[0066] IFN-α-Zn 2+ Preparation of complex micropowder: 10 mg of PEG6000, 4 mg of IFN-α and 2 mg of protective agent zinc carbonate were dispersed in 1 ml of double distilled water, mixed by vortex for about 3 minutes, after freeze-drying, washed with acetonitrile and centrifuged to remove PEG6000 to obtain IFN-α- Zn 2+ Complex powder.
[0067] Interferon suspension (S / O 1 ) preparation: freeze-dried zinc ion-stabilized interferon (IFN-α-Zn 2+ ) p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com