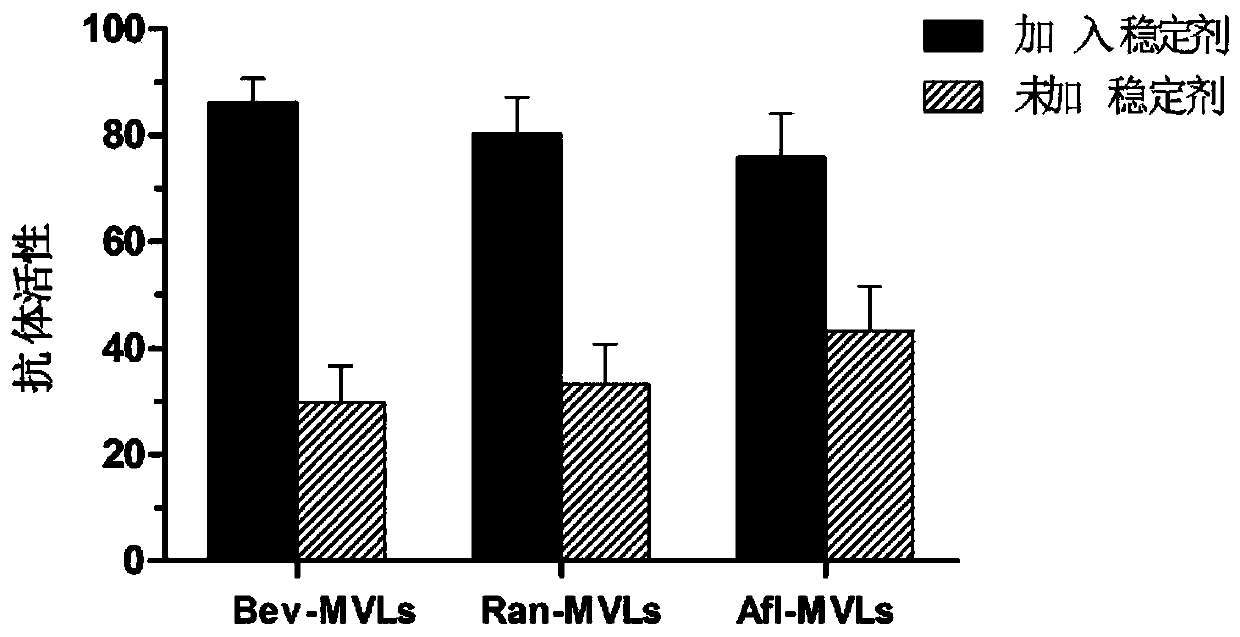
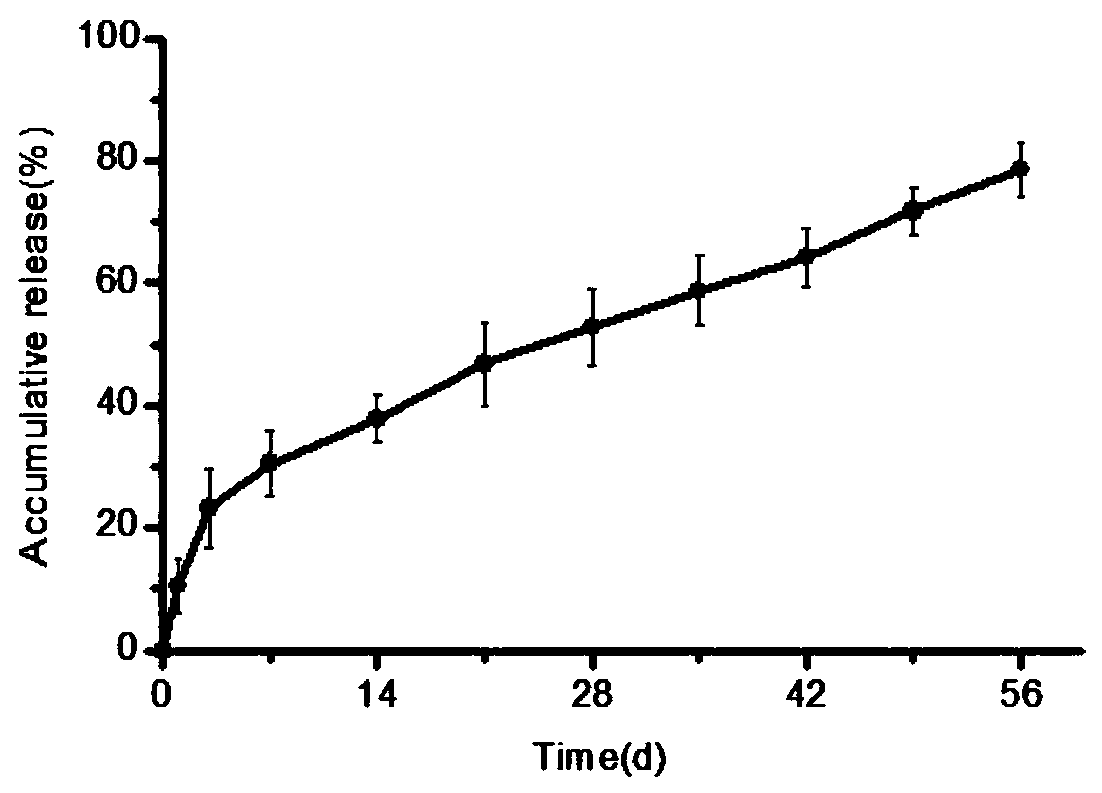
Polycystic liposome gel capable of overcoming burst release and maintaining antibody activity and preparation method thereof
A technology of multivesicular liposomes and antibody activity, which is applied in the direction of liposome delivery, antibodies, medical preparations of non-active ingredients, etc. It can solve problems such as inflammation and irritation, and achieve improved compliance, prolonged release time, Reduce the effect of drug burst release phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of multivesicular liposome gel sustained-release preparation loaded with bevacizumab (LG1):
[0053] Bevacizumab multivesicular liposome (Bev-MVLs) preparation: the bevacizumab solution of 25mg / mL that contains 3mL 6% sucrose and 10% (w / v) HSA is used as internal aqueous phase; Accurately weigh Dioleoylphosphatidylcholine, dipalmitoylphosphatidylglycerol, cholesterol, triolein 23mg, 7mg, 19mg, 7mg respectively in 10ml beaker, add 3ml chloroform to dissolve, obtain lipid phase; 10000rpm high-speed shearing conditions were added dropwise to the lipid phase, and sheared for 5min to form w / o type colostrum; the colostrum was added dropwise to 12mL of 4% glucose and 60mmol under 4000rpm high-speed shearing conditions. / L of lysine in the external aqueous phase solution, keep for 1min to form a w / o / w type double emulsion; 37°C rotary evaporation to obtain multivesicular liposome suspension;
[0054] Preparation of multivesicular liposome gel sustained-release pre...
Embodiment 2
[0056] Preparation of multivesicular liposome gel sustained-release formulation loaded with ramuzumab:
[0057] Rabluzumab multivesicular liposomes (Ran-MVLs) preparation: 2 mL of 25 mg / mL of Rabluzumab solution containing 6% sucrose and 10% (w / v) HSA was used as the internal aqueous phase; Oleoylphosphatidylcholine, dipalmitoylphosphatidylglycerol, cholesterol, triolein 14mg, 5mg, 13mg, 5mg respectively in a 10ml beaker, add 2ml chloroform and ether mixed solution (1:1, v / v) Dissolve to obtain a lipid phase; add the inner water phase dropwise to the lipid phase under 10000rpm high-speed shear conditions, and shear for 5min to form w / o type colostrum; put the colostrum under 4000rpm high-speed shear conditions Add dropwise to 9mL of 5% glucose and 40mmol / L lysine in the external aqueous phase solution, keep for 1min to form w / o / w type double emulsion; 37°C rotary evaporation to obtain multivesicular liposome suspension liquid;
[0058] Preparation of multivesicular liposome ...
Embodiment 3
[0060] Preparation of multivesicular liposome gel sustained-release formulation loaded with aflibercept:
[0061] Preparation of aflibercept multivesicular liposomes (Afl-MVLs): 2 mL of 10 mg / mL aflibercept solution containing 5% sucrose and 10% (w / v) HSA was used as the internal aqueous phase; Oleoylphosphatidylcholine, dipalmitoylphosphatidylglycerol, cholesterol, triolein 15mg, 6mg, 15mg, 6mg respectively in a 10ml beaker, add 2ml chloroform and ether mixed solution (1:1, v / v) Dissolve to obtain a lipid phase; add the inner water phase dropwise to the lipid phase under 11000rpm high-speed shear conditions, and shear for 6min to form w / o type colostrum; put the colostrum under 6000rpm high-speed shear conditions Add dropwise to 8mL of 4% glucose and 40mmol / L lysine in the external aqueous phase solution, keep for 1min to form a w / o / w type double emulsion; 37°C rotary evaporation to obtain multivesicular liposome suspension liquid;
[0062] Preparation of multivesicular lip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com