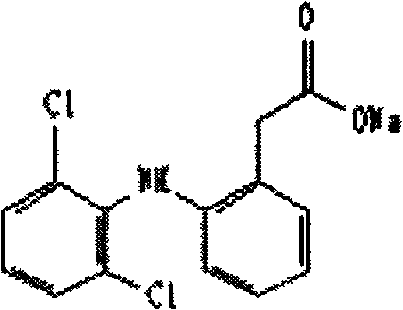
Diclofenac sodium sustained release tablet and preparation method thereof
A technology of diclofenac sodium and sustained-release tablets, which is used in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc. Good release profile and good formulation stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
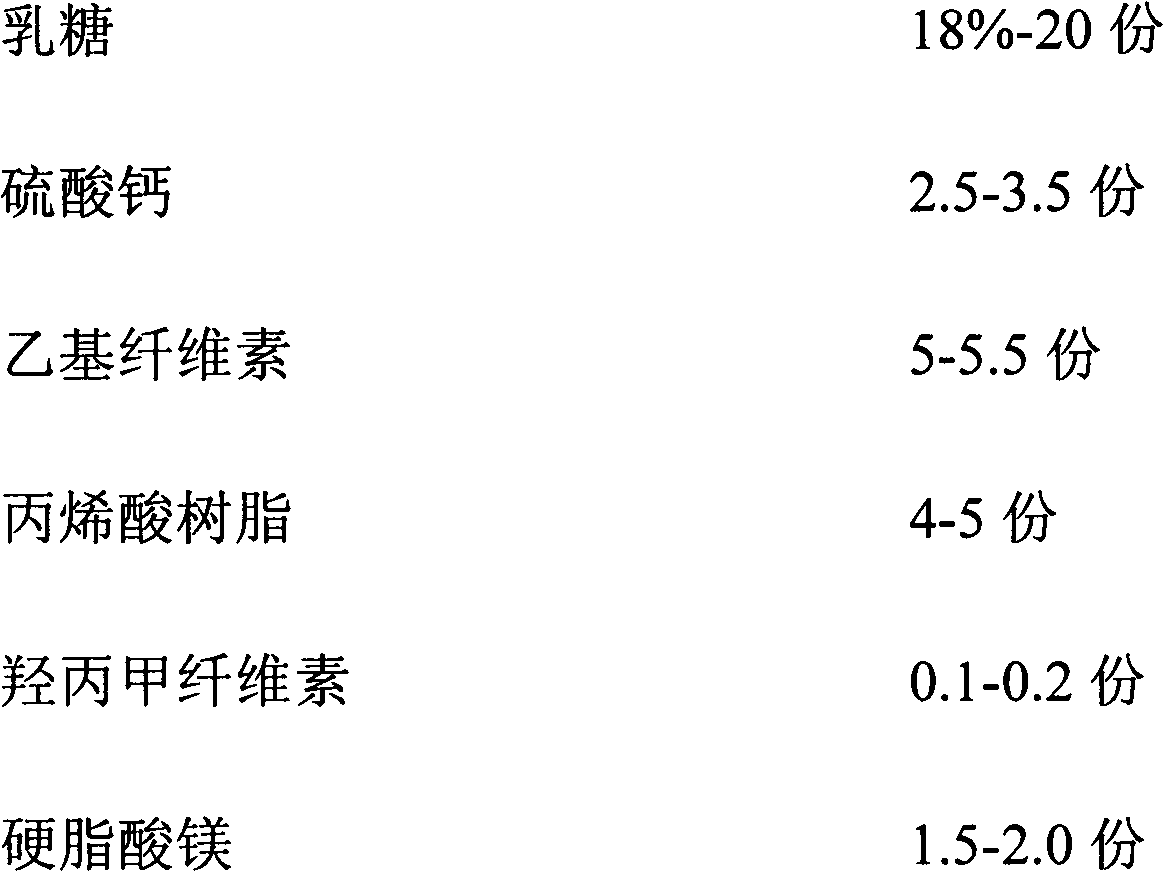
[0024] Diclofenac sodium, lactose, calcium sulfate, ethyl cellulose, acrylic resin, hypromellose, and magnesium stearate were weighed according to the amounts in Table 1.
[0025] Get the above-mentioned amount of diclofenac sodium, pass through a 60-mesh sieve, get the described amount of lactose and calcium sulfate, pass through an 80-mesh sieve, get the described amount of ethyl cellulose, pass through a 100-mesh sieve for pulverization, and pass through an 80-mesh sieve.
[0026] The above-mentioned amount of acrylic resin and hypromellose were dissolved in 95% ethanol.
[0027] Mix the above diclofenac sodium, calcium sulfate, lactose and ethyl cellulose evenly, add acrylic resin, hypromellose and 95% ethanol binder to make a soft material, granulate with 18-mesh nylon mesh, and ventilate at 70°C Dried, granulated with 18 mesh wire mesh, mixed with the above-mentioned amount of magnesium stearate, compressed into tablets, and packaged to obtain the diclofenac sodium susta...
Embodiment 2-6
[0029] Except that the amount of each raw material is weighed according to Table 1, other method steps are all the same as in Example 1. The diclofenac sodium sustained-release tablets of Examples 2-6 were prepared.
[0030] Table 1
[0031]
[0032] Wherein, all numerical values are the dosage of diclofenac sodium per 1000 tablets, and the unit is g.
[0033] The beneficial effect of the technical solution provided by the present invention will be illustrated through experiments below.
[0034] Determination of release: get this product, according to release assay (Chinese Pharmacopoeia edition in 2000 two appendix X D first method), adopt the device of dissolution assay (Chinese Pharmacopoeia edition two appendix XC first method in 2000), with phosphoric acid 900ml of salt buffer solution (pH value 7.4) is the solvent, and the rotating speed is 100 revolutions per minute. Operate according to the law. Take 5ml of the solution for filtration in 2 hours, 6 hours, and 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com