Vesicle for enhancing skin penetration and preparation method of same
一种囊泡、使用量的技术,应用在护理皮肤的制剂、梳妆用配制品、含有效成分的医用配制品等方向,能够解决损伤角质层细胞间脂质结构、不能被视为化学促渗剂等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and comparative example 1 to 3
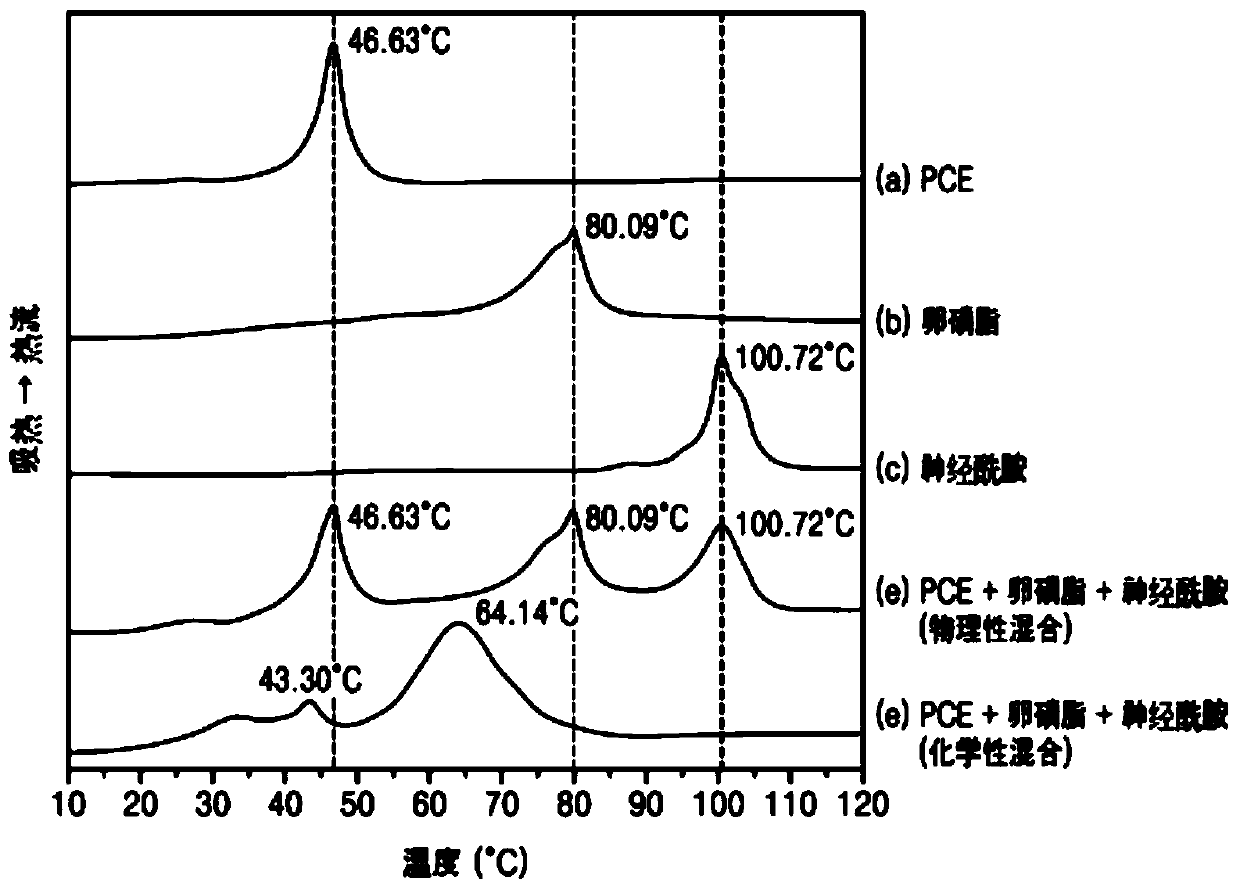
[0050] Example 1 and Comparative Examples 1 to 3: Preparation of Vesicles Including Skin Absorption-Promoting Components
[0051] The vesicles of Example 1 and Comparative Examples 1 to 3 were prepared using different skin absorption promoting ingredients from each other. Specifically, vesicles were prepared by heating to 80°C to 90°C and dissolving Item B slowly poured into Item A at room temperature. Example 1 included ceramide as a skin absorption promoting ingredient, Comparative Example 1 did not include a skin absorption promoting ingredient, Comparative Example 2 included menthol as a skin absorption promoting ingredient, and Comparative Example 3 included ethanol as a skin absorption promoting ingredient.
[0052] The specific components and contents of the compositions prepared according to Example 1 and Comparative Examples 1 to 3 are shown in Table 1 below. Unless otherwise stated in this specification, the content of ingredients is % by weight.
[0053] [Table 1]...
Embodiment 2 to 6
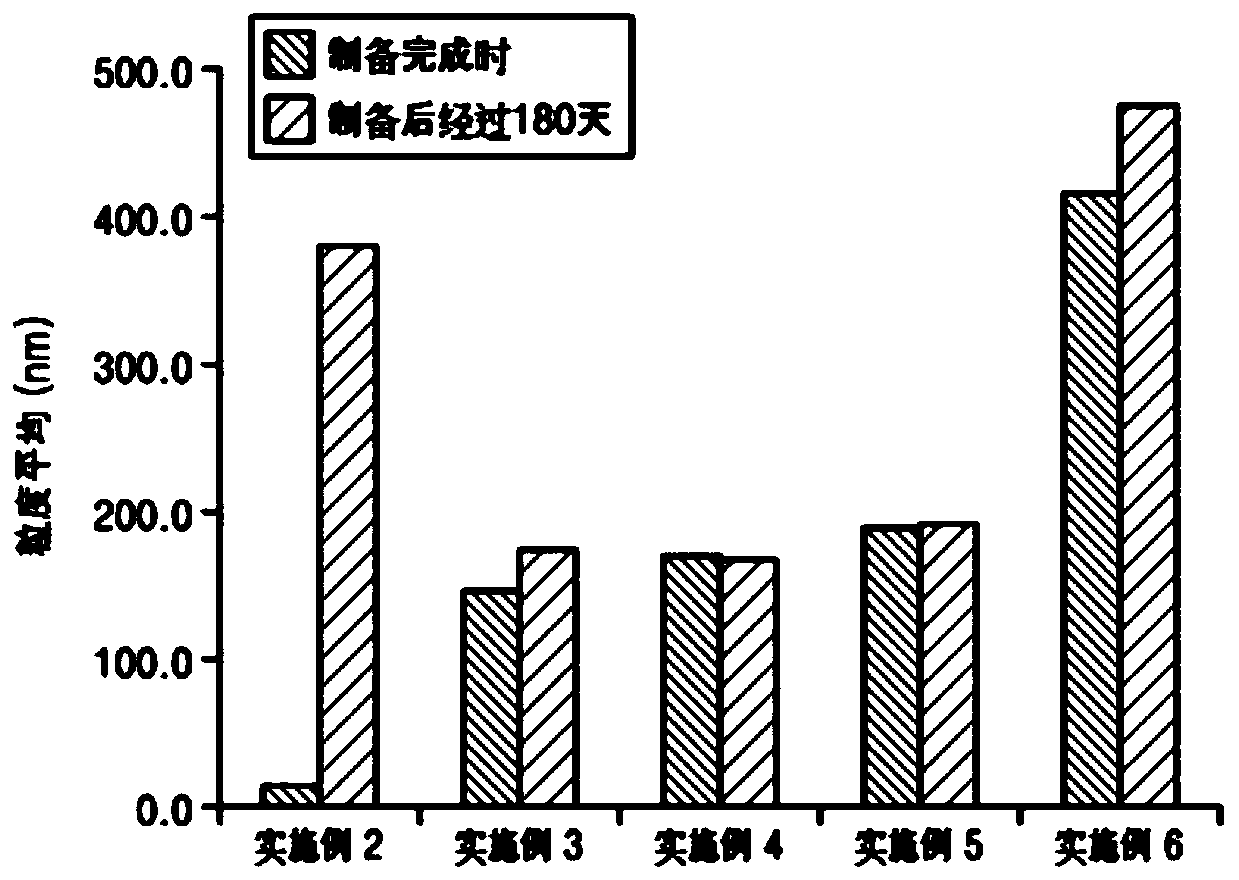
[0061] Examples 2 to 6: Preparation of ceramide-containing vesicles
[0062] In order to stabilize ceramide in the dosage form, as shown in Table 3, the dosage forms of Examples 2 to 6 were prepared with different contents of polyoxyethylenecholesteryl ether (PCE) and hydrogenated lecithin. Specifically, the dosage form is prepared by slowly pouring into item B at room temperature after heating to 80°C to 90°C and dissolving item A.
[0063] [table 3]
[0064]
Embodiment 7
[0085] Example 7 and Comparative Example 4: Preparation of a ceramide-containing vesicle dosage form for testing the effect of promoting skin absorption
[0086] In order to examine the skin absorption promoting effect by said Test Example 4 with respect to the dosage form of Example 5 in which the effect of stabilizing ceramide in the dosage form was demonstrated, a vesicle dosage form was prepared with the ingredients and contents shown in Table 5 below. Retinol was used as a model drug. Specifically, after heating item A to 80°C to 90°C and dissolving it, a vesicle dosage form is prepared by slowly adding item B at room temperature. The only difference between Comparative Example 4 and Example 7 is the content of ceramide. That is, it is composed of the above ingredients to confirm how much influence ceramide has as a skin absorption enhancer.
[0087] [table 5]
[0088]
[0089]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com