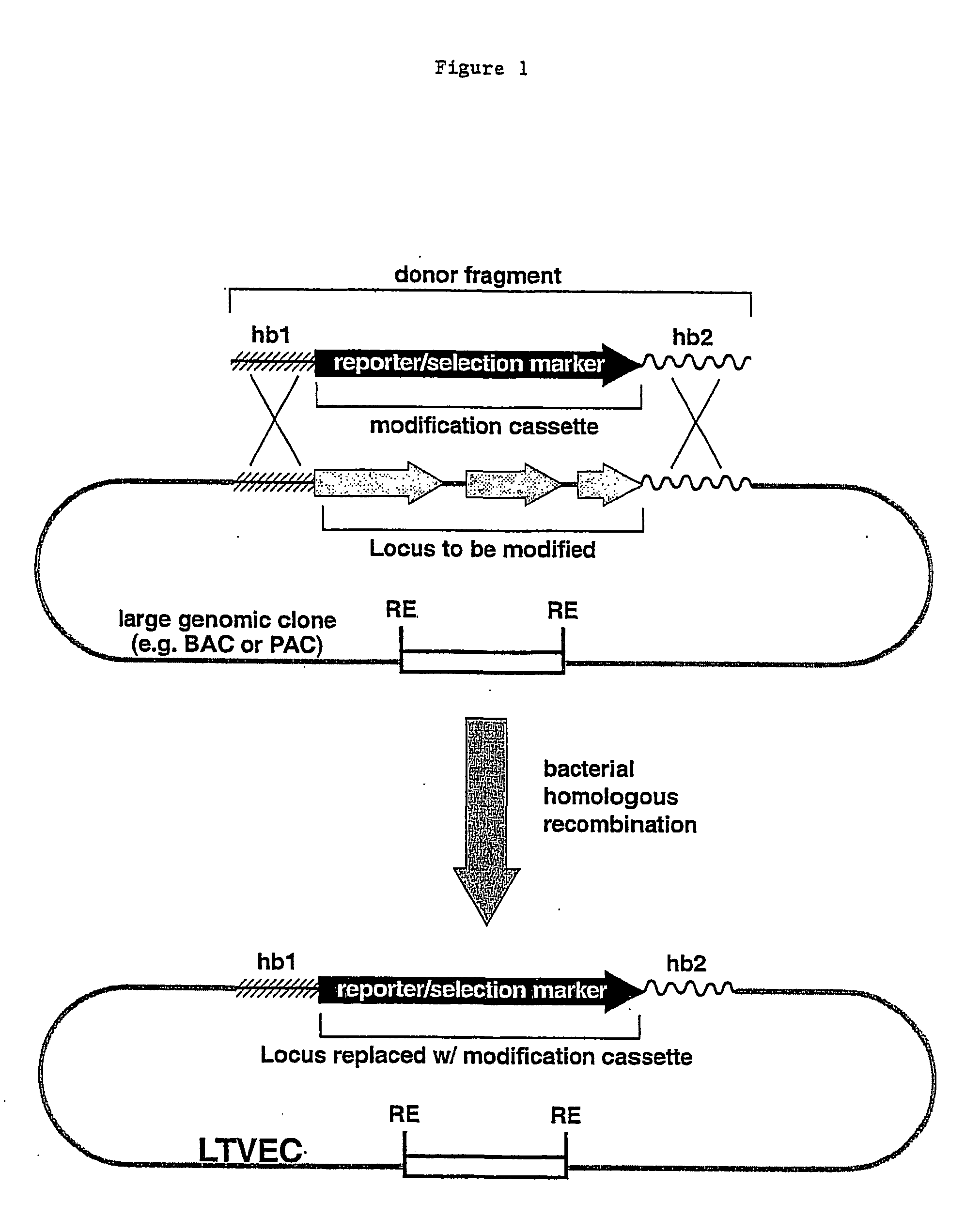
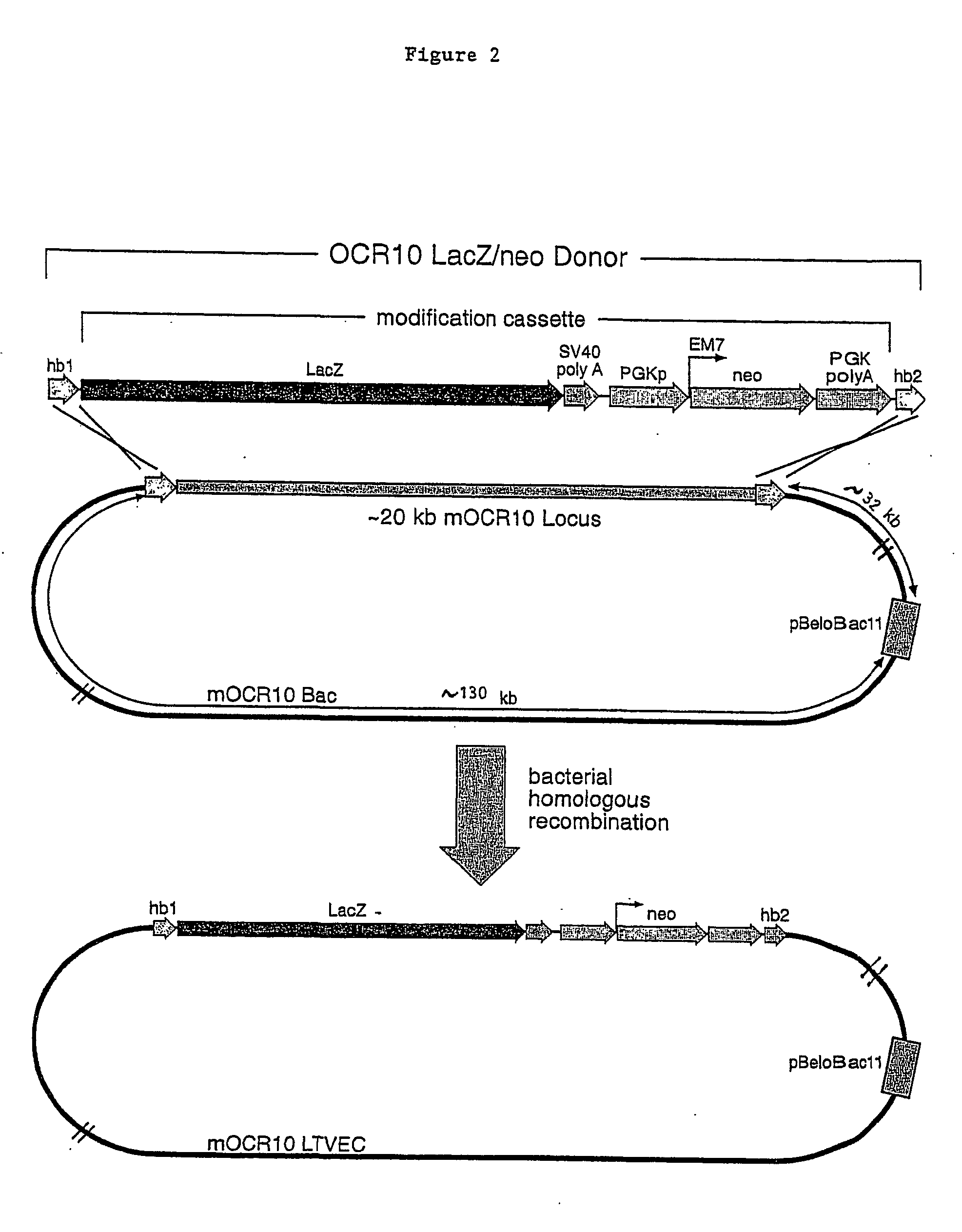
Methods of modifying eukaryotic cells
a technology of eukaryotic cells and vectors, applied in the field of methods of modifying eukaryotic cells, can solve the problems of reducing any potential benefit, vectors have not been generally useful for modifying endogenous genes or chromosomal changes,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Engineering Mouse ES Cells Bearing a Deletion of the OCR10 Gene
a. Selection of a Large Genomic DNA Clone Containing mOCR10.
[0121] A Bacterial Artificial Chromosome (BAC) clone carrying a large genomic DNA fragment that contained the coding sequence of the mouse OCR10 (mOCR10) gene was obtained by screening an arrayed mouse genomic DNA BAC library (Incyte Genomics) using PCR. The primers employed to screen this library were derived from the mOCR10 gene cDNA sequence.
[0122] Two primer pairs where used:
(a) OCR10.RAA(5′-AGCTACCAGCTGCAGATGCGGGCAG-3′)andOCR10.PVIrc(5′-CTCCCCAGCCTGGGTCTGAAAGATGACG-3′)which amplifies a 102 bp DNA; and(b) OCR10.TDY(5′-GACCTCACTTGCTACACTGACTAC-3′)andOCR10.QETrc(5′-ACTTGTGTAGGCTGCAGAAGGTCTCTTG-3′)which amplifies a 1500 bp DNA.
[0123] This mOCR10 BAC contained approximately 180 kb of genomic DNA including the complete mOCR10 coding sequence. This BAC clone was used to generate an LTVEC which was subsequently used to delete a portion of the coding region of...
example 2
Increased Targeting Frequency and Abrogation of the Need to Use Isogenic DNA when LTVECs are Used as the Targeting Vectors
[0136] As noted above, the increased targeting frequency obtained using long homology arms should diminish the benefit, if any, derived from using genomic DNA in constructing LTVECs that is isogenic with (i.e. identical in sequence to) the DNA of the eukaryotic cell being targeted. To test this hypothesis, Applicants have constructed several LTVECs using genomic DNA derived from the same mouse substrain as the eukaryotic cell to be targeted (presumably isogenic), and a large number of other LTVECs using genomic DNA derived from mouse substrains differing from that of the eukaryotic cell to be targeted (presumably non-isogenic). The non-isogenic LTVECs exhibited an average targeting frequency of 6% (ranging from 1-20%, Table 1), while the isogenic LTVECs exhibited as average targeting frequency of 3% (ranging from 2-5%), indicating that the rate of successful tar...
example 3
Detailed Description of the TagMan®-Based MOA for Identification of Targeted ES Clones
[0137] ES cell clones that have taken up the LTVEC and incorporated it into the genome at the targeted locus by homologous recombination are identified by a modification of allele (MOA) assay that uses real-time quantitative PCR to discern the difference between targeted ES cell clones, in which one of the two targeted alleles is modified, and non-targeted ES cell clones, in which both alleles remain unmodified. The MOA assay consists of a primary and a secondary screen. The primary screen contains the following steps: (1) growth of LTVEC-transfected ES cell clones on gelatin-coated 96-well plates; (2) isolation of genomic DNA from each ES cell clone; (3) use of each genomic DNA sample as a template in 8 separate quantitative PCRs on two 384-well plates in which 2 of the PCRs employ a target-locus-specific primer set that hybridyzes to DNA sequences at one end of the genomic fragment targeted for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| targeting frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com