Human antibodies derived from immunized xenomice
a technology of human antibodies and xenomice, which is applied in the field of immunology, can solve problems such as problems in the use of human antibodies in vivo diagnostics, in particular, and in the treatment of human diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
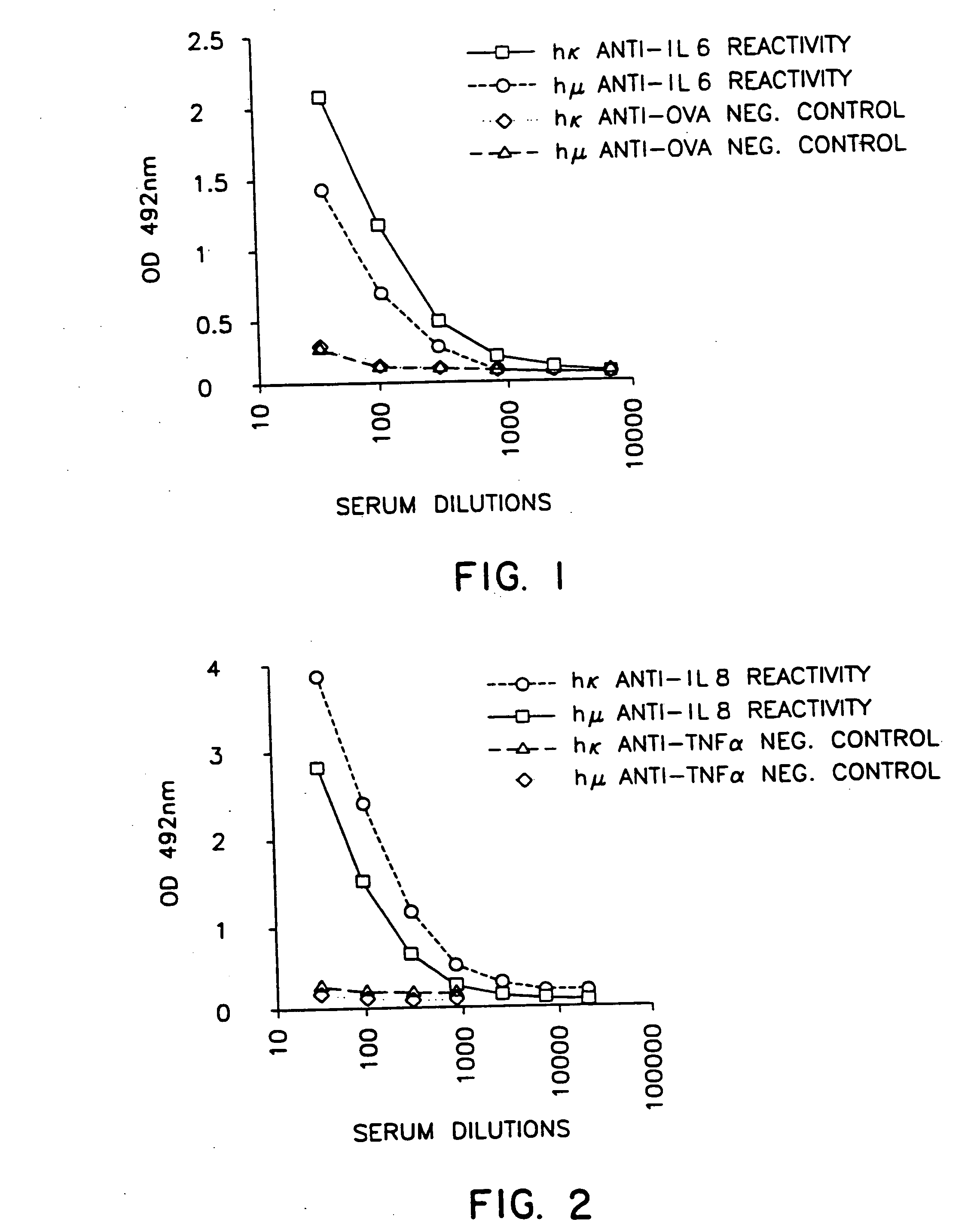
Human Antibodies Against Human IL-6
[0075] Three to 5 xenomice aged 8-20 weeks were age-matched and immunized intraperitoneally with 50 μg human IL-6 emulsified in complete Freund's adjuvant for primary immunization and in incomplete Freund's adjuvant for subsequent injections. The mice received 6 injections 2-3 weeks apart. Serum titers were determined after the second dose and following each dose thereafter. Bleeds were performed 6-7 days after injections from the retrobulbar plexus. The blood was allowed to clot at room temperature for about 2 hours and then incubated at 4° C. for at least 2 hours before separating and collecting the sera.
[0076] ELISAs were conducted as described above by applying 100 μl per well of recombinant human IL-6 at 2 mg / ml in coating buffer. Plates were then incubated at 4° C. overnight or at 37° C. for 2 hours and then washed three times in washing buffer. Addition of 100 μl / well blocking buffer was followed by incubation at room temperature for 2 hou...
example 2
Human Antibodies Against Human IL-8
[0079] Immunization and serum preparation were as described in Example 1 as except that human recombinant IL-8 was used as an immunogen.
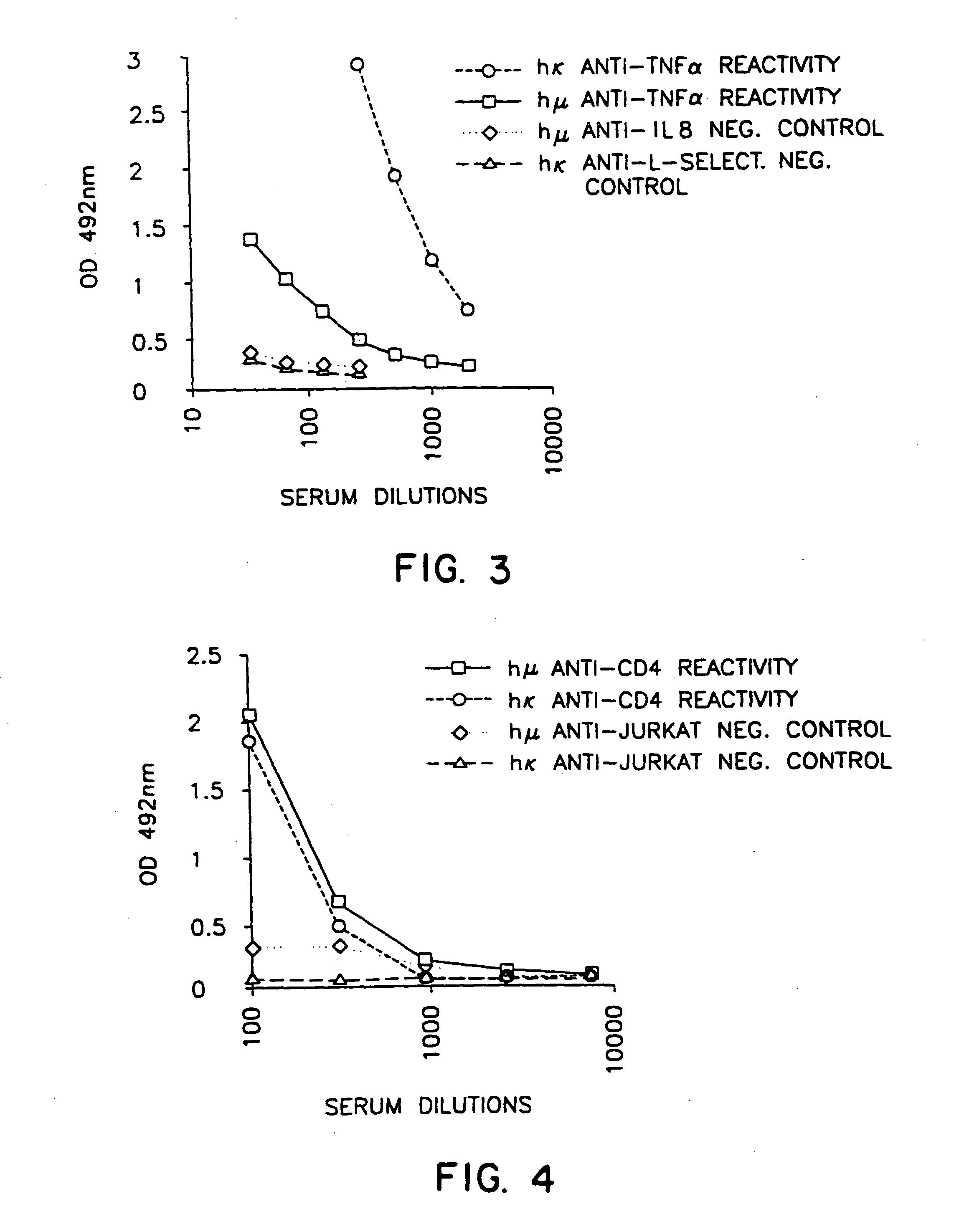
[0080] ELISA assays were performed with respect to the recovered serum, also exactly as described in Example 1, except that the ELISA plates were initially coated using 100 μl / well of recombinant human IL-8 at 0.5 mg / ml in the coating buffer. The results obtained for various serum dilutions from xenomouse A260-5 after 6 injections are shown in FIG. 2. Human anti-IL-8 reactivity was again shown at serum dilutions having concentrations higher than that represented by a 1:1,000 dilution.
example 3
Human Antibodies Against Human TNFα
[0081] Immunization and serum preparation were conducted as described in Example 1 except that human recombinant TNFα was substituted for human IL-6. ELISAs were conducted as described in Example 1 except that the initial coating of the ELISA plate employed 100 μl / well recombinant human TNFα at 1 mg / ml in coating buffer.
[0082] The dilution curves for serum from xenomouse A210-8 after 6 injections obtained are shown in FIG. 3. Again significant titers of human anti-TNFα reactivity were shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com