Anti-HER2 dual-specific antibody and application thereof
A bispecific antibody, HCDR2 technology, applied in the field of biomedicine, can solve problems such as poor stability, poor binding activity, and precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Construction and expression of bispecific antibodies against different epitopes of human HER2
[0055] DNA fragments encoding trastuzumab heavy chain (including knob structure), pertuzumab heavy chain (including hole structure) and trastuzumab light chain (SEQ ID NO: 2) were synthesized from the whole gene and cloned into The expression vector constructed in our laboratory consists of the following components:
[0056] 1) glutamine synthetase gene, as a selection marker; or neomycin resistance gene, as a heavy chain selection marker,
[0057] 2) Origin of replication: ori,
[0058] 3) origin of replication from vector pUC18, which allows replication of this plasmid in E. coli,
[0059] 4) a β-lactamase gene which confers ampicillin resistance in Escherichia coli,
[0060] 5) Immediate early enhancer and promoter from human cytomegalovirus,
[0061] 6) a human 1-immunoglobulin polyadenylation ("poly A") signal sequence, and
[0062] As described above, im...
Embodiment 2
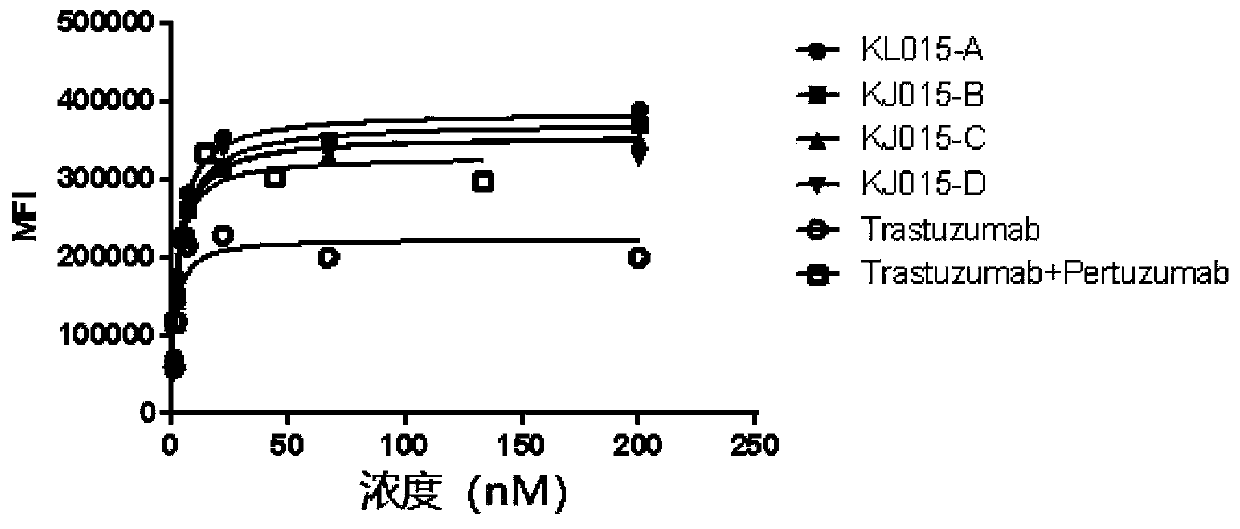
[0067] Example 2: Determination of the affinity between anti-human HER2 different epitope bispecific antibodies and HER2
[0068] Biacore T200 was used to determine the affinity of single-target HER2 antibodies trastuzumab, pertuzumab, and HER2 bispecific antibody KJ015w to three forms of HER2 antigen.
[0069] First, the Anti-Human Fc (AHC) antibody was coupled to the CM5 chip, and then the antibody IgG (2 μg / ml) to be tested was captured by AHC, and three kinds of antigen molecules of different concentrations flowed over the chip surface captured IgG, The binding activity of HER2 antibody to different concentrations of antigens was detected, and the obtained data were fitted according to Biacore T200 analysis software to obtain exact kinetic constants. The comparison results are shown in Table 2.
[0070] Table 2: Affinity test results of antibody KJ015w and three forms of HER2 antigen
[0071]
[0072] Trastuzumab, Pertuzumab and the bispecific anti-HER2 antibody KJ015w...
Embodiment 3
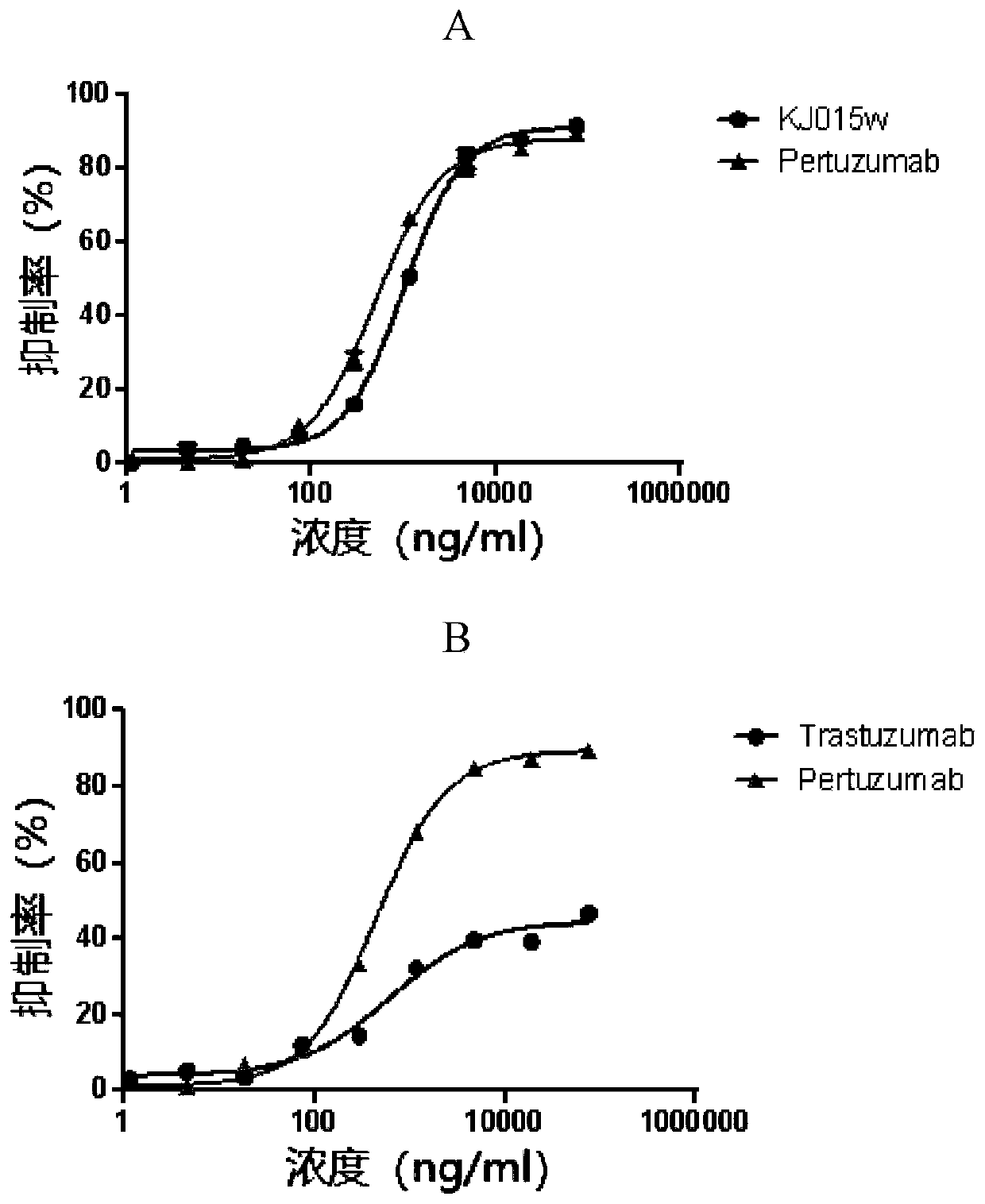
[0073]Example 3: Detection of anti-human HER2 different epitope bispecific antibody cell proliferation inhibitory activity
[0074] MDA-MB-175 cell culture flasks were placed in a 37°C, 5% carbon dioxide constant temperature incubator for static culture, and the color of the medium and cell confluence were observed every day, and the cell doubling time was recorded. Cells were subcultured every 2-4 days. When the cells are about 80% full layer, the seed plate is digested. Discard the medium, wash once with PBS, and add 0.25% trypsin (Gibco) for digestion. Collect the cells into a centrifuge tube by pipetting and centrifuge at 500 g for 3 min. Discard the supernatant, add complete medium to resuspend the cells, and take 100 μL of the cell suspension for counting. Adjust the cell density to 1 × 10 with medium containing 2% FBS 5 cells / mL, 100 μL per well was spread into a 96-well plate.
[0075] Dilute the drug to be tested with medium containing 2% FBS, first dilute the dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com