Anti-Ebola virus monoclonal antibody, its preparation method and use
A monoclonal antibody, Ebola virus technology, applied in the fields of immunology and molecular biology, to achieve high ADCC activity, good antigen binding activity and virus neutralization activity, and good clinical application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Antibody MIL77 nucleotide sequence obtained
[0128] The antibody amino acid sequence is recombinant antibody sequence, from Patent WO2009 / 094755 A1 and US2004 / 0053865A1. The reverse translation was selected in mammalian cells in mammalian cells, and the nucleotide sequence of the antibody MIL77 of the present invention was obtained by rational optimization of bioinformatics techniques, using molecular modeling and molecular biological mutation experiment.
[0129] Among them, the sequence of light chain nucleotides of the antibody MIL77-1 is:
[0130] GacatccagatgactcagtctccccccccctatctgtatctgtggggagaActGtctCccatcacatg Tcgagcaagtgagaatttacagtagttagcatgtatcagcagaaacagggaaaatctcctcagctcctgtc Tattctgcaacaatcttagcagatgtgtgccatcaaggtcagtggcagtgatcaggcactcagtattccccca AgatcaacagcctgcAgtctGaAgatttgggctTttctTctGtcaAcatttgggggTCCGTCGTTCGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGGTCGTCCCGTTTTGGGGTCCCCGTTTTGGGGTACTCCCGTCGTTCGGGGGG AggggGgaccaagctggaaataaaaa cgtacggtggctgcacca...
Embodiment 2
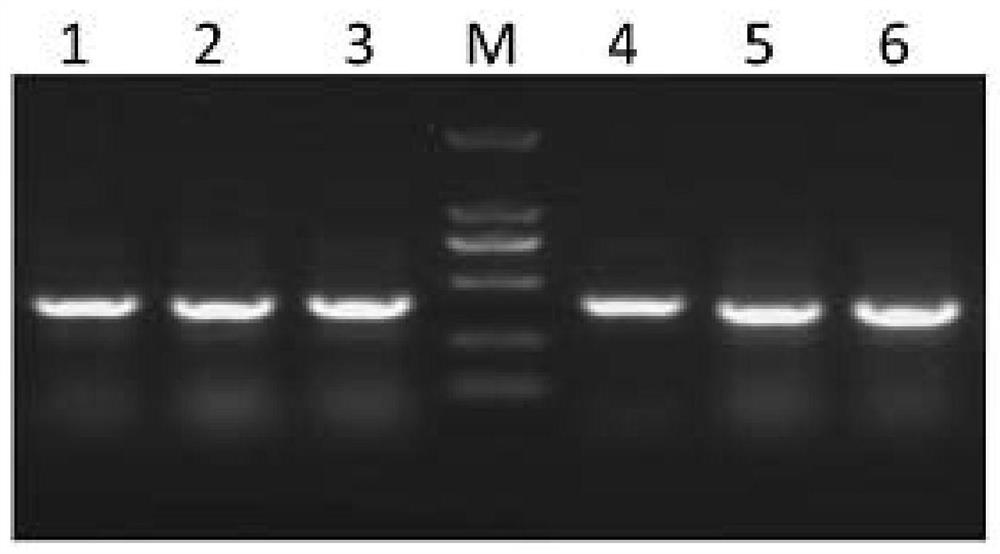
[0153] Example 2: Cloning of antibody MIL77-1, MIL77-2 and MIL77-3 gene
[0154] Experimental material
[0155] PHUSION polymerase, TAQ DNA polymerase, restriction endonuclease, PGEM-T-Easy vector, Pyrobest Dnapolymese is NEB company products;
[0156] DNTP, DNA Marker standard is TAKARA products;
[0157] DNA Recycling Kit is QIAGEN's products;
[0158] TRANS2-Blue feels the full gold company product;
[0159]Small-to-medium plasmid kit (DP107-02) is the product of Tiangen biochemical company;
[0160] The large triump kit (DP117) is the product of the Tiangen biochemical company;
[0161] OPD is Sigma products;
[0162] Fitc marked sheep anti-human antibody: for PIERCE products;
[0163] Primer design is Biosun software;
[0164] Bimget Synthesis (GENEWIZ), Jin Weizhi Biotechnology (Beijing) Co., Ltd.
[0165] Gene sequencing is done by Beijing Noris Genetory Research Center Co., Ltd.
[0166] 2. Experimental methods and results
[0167] The lightweight chain variable region ge...
Embodiment 3
[0193] Example 3: Preparation of MIL77-1, MIL77-2 and MIL77-3 antibodies
[0194] Experimental material
[0195] The dry powder medium for entrusted Hyclone processing was prepared, and the cells were used to cultivate cells during the main cells, cell line screening and antibody preparation process, and the cell screening was added to the configured medium (Methionine Sulfoximine) (Methionine Sulfoximine) from SIGMA , MSX), used to prepare a cell count after formulating a solution from Sigma.
[0196] 2. Experimental methods and results
[0197] 2.1 Construction of antibody eukaryotic expression vector
[0198] The expression vector PTGS-FRT-DHFR (patent authorization number: ZL2005100643335.0) obtained by the Applicant is selected, and the hygromycin selection label is removed, and GS (Glutamine Synthetase, Glutamine Synthetase) is added to the PSHA1 and XHO1. Expressing the box as a screening mark; where GS cDNA is obtained from the cell line CHO expression GS through RT-PCR. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com