crRNA target point and CRISPR-Cas13a system for detecting Ebola virus
An Ebola virus and target sequence technology, applied in the field of crRNA target and CRISPR-Cas13a system, can solve the problems of complex sample processing, high instrument requirements, and insufficient sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
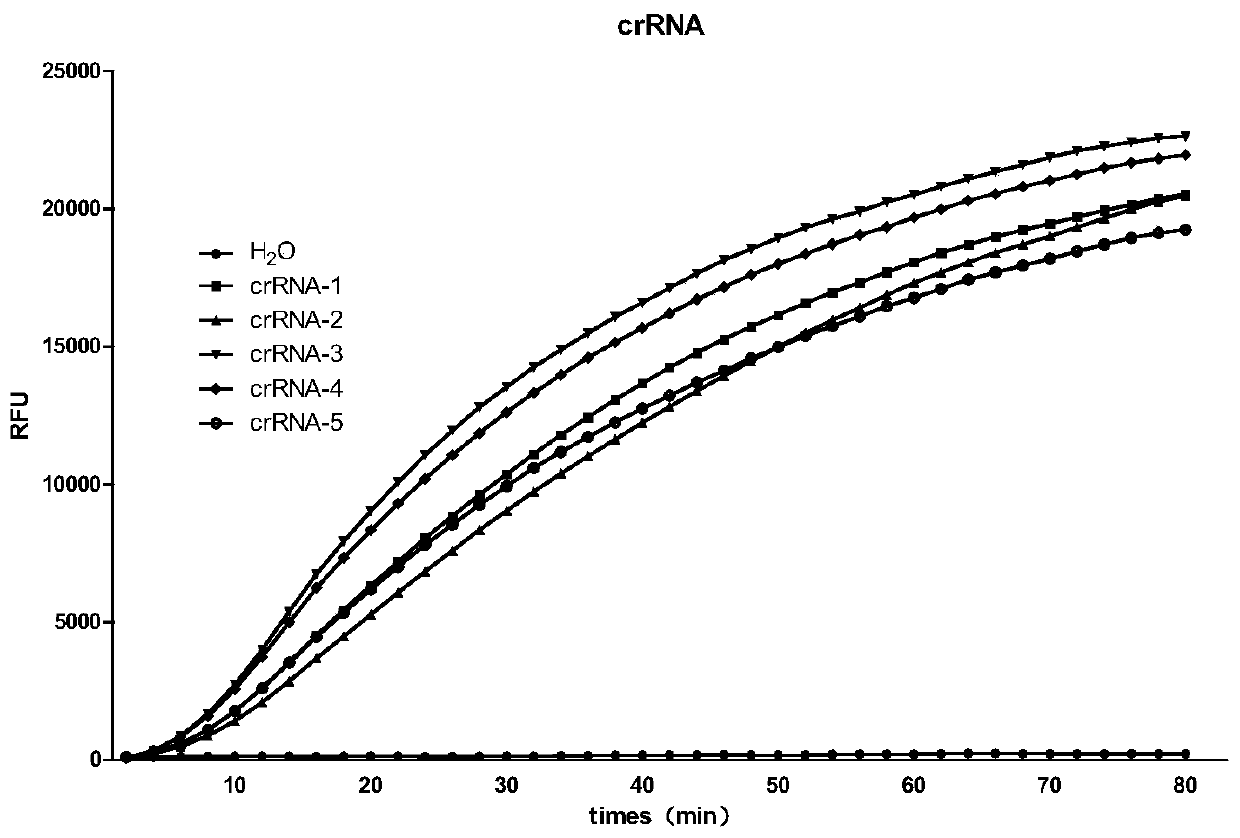
Embodiment 1
[0050] Example 1, Ebola virus nucleic acid detection kit and detection method based on CRISPR-Cas13a system
[0051] (1) Ebola virus nucleic acid detection kit based on CRISPR-Cas13a system
[0052] 1. LwCas13a protein
[0053] For the expression, purification and activity identification of LwCas13a protein, refer to the method in the patent document with the title of invention "An effective Cas13a-based anti-dengue virus nucleic acid target and its application" and the publication number is CN108715849A. Specific steps are as follows:
[0054] 1. Induced expression, purification and identification of LwCas13a protein
[0055] The LwCas13a expression plasmid Addgene-PC013-Twinstrep-SUMO-huLwCas13a was obtained from the Addgene platform, and the LwCas13a expression plasmid was transferred into Rosetta (DE3) competent cells, cultured in TB liquid medium at 37°C and 200rpm for more than 14 hours, and inserted into new Amp + In the resistant TB medium, culture at 37°C and 300r...
Embodiment 2
[0123] Embodiment 2, the sensitivity detection of the inventive method
[0124] The plasmid standard after serial dilution was used as a template, and the plasmids containing EBOV gene fragments in different concentrations were detected according to the method in Example 1, so as to test the sensitivity of the method of the present invention. Specific steps are as follows:
[0125] 1. Carry out gradient dilution to the plasmid standard substance obtained in Step (1) of Example 1 to obtain plasmid solutions containing different concentrations of EBOV gene fragments: 10 6 copies / μL, 10 5 copies / μL, 10 4 copies / μL, 10 3 copies / μL, 10 2 copies / μL, 10 1 copies / μL, 10 0 copies / μL.
[0126] 2. Carry out RAA amplification according to the method in Step (1)-4 of Example 1 to obtain the RAA amplification product.
[0127]3. After being amplified by RAA, take 5 μL of the amplified product to detect Ebola virus nucleic acid based on the CRISPR-Cas13a system according to the metho...
Embodiment 3
[0129] Embodiment 3, the specific detection of the method of the present invention
[0130] Forest encephalitis virus (TBEV), dengue virus type 2 (DENV2), dengue virus type 4 (DENV4), Japanese encephalitis virus (JEV), yellow fever virus (YFV), HBV virus (HBV) and Bennett virus Coxella speii (Cb) pathogenic nucleic acid was used as a template, and different viral nucleic acids were detected according to the method in Example 1, to verify the specificity of the method of the present invention. Specific steps are as follows:
[0131] 1. Ebola virus (EBOV), forest encephalitis virus (TBEV), dengue virus type 2 (DENV2), dengue virus type 4 (DENV4), Japanese encephalitis virus (JEV), yellow fever virus ( YFV), HBV virus (HBV) and Coxella benardii (Cb) nucleic acids were used as detection templates, and RAA amplification was performed according to the method in step (1) of Example 1, to obtain RAA amplification products.
[0132] 2. After being amplified by RAA, take 5 μL of the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com