Methods for sterilizing preparations containing albumin
A technology of albumin and preparation, applied in the field of preparations containing albumin by radiation method, which can solve the problems of loss of vitality and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0061] A first preferred embodiment of the present invention relates to a method for sterilizing preparations containing radiation-sensitive albumin comprising: applying suitable radiation to effectively sterilize the material and to protect the material from radiation damage Rate is the time for which irradiated energy in preparations containing albumin is effective to sterilize the material.
[0062] Another preferred embodiment of the present invention relates to a method for sterilizing a radiation-sensitive albumin-containing preparation, said method comprising: (i) adding at least one stabilizer to effectively protect said albumin-containing preparation A radiation-protective amount is added to the albumin-containing formulation; and (ii) irradiating said albumin-containing formulation with radiation at an effective rate for a time effective to sterilize the material.
[0063] Another preferred embodiment of the present invention relates to a method for sterilizing a rad...
Embodiment 1
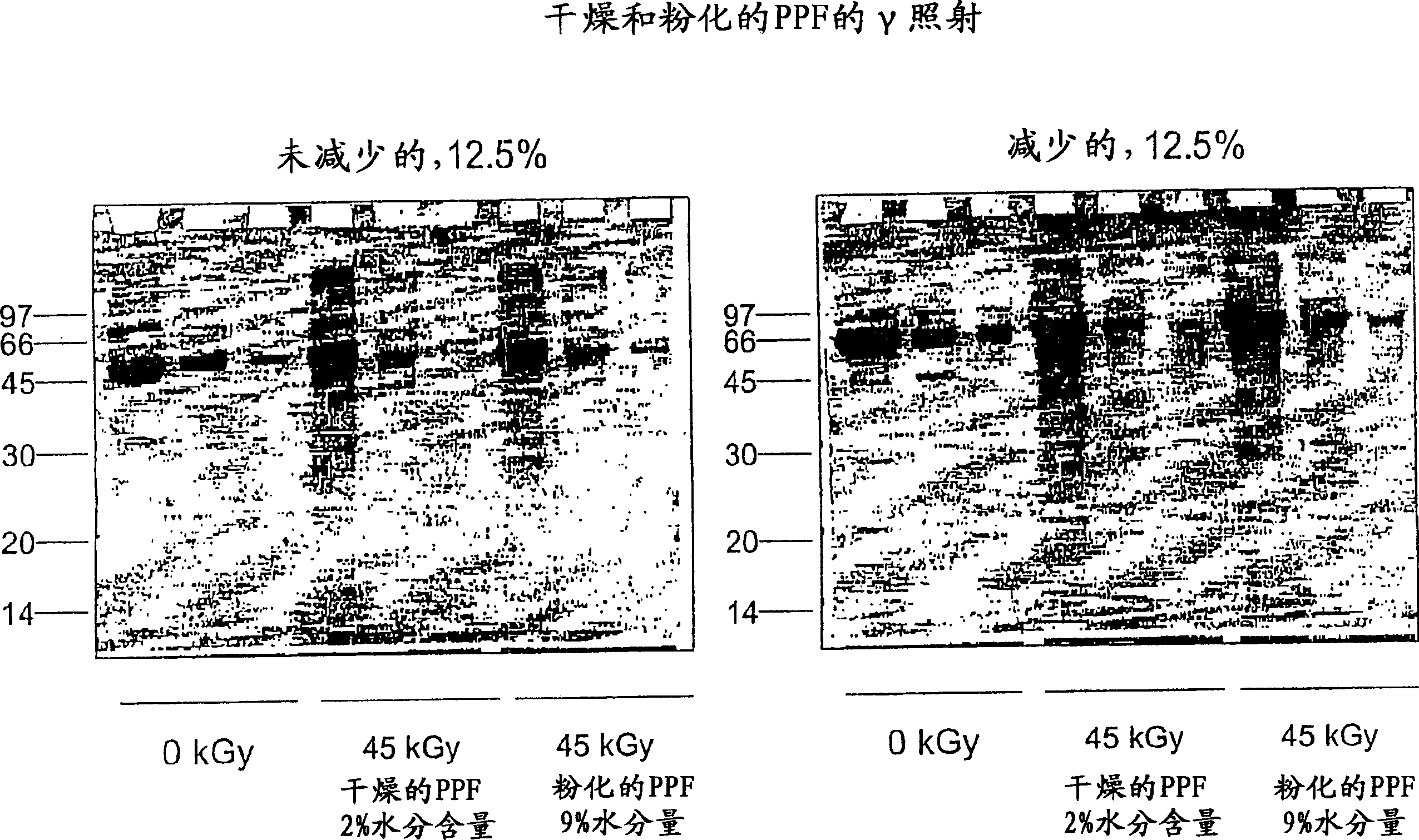
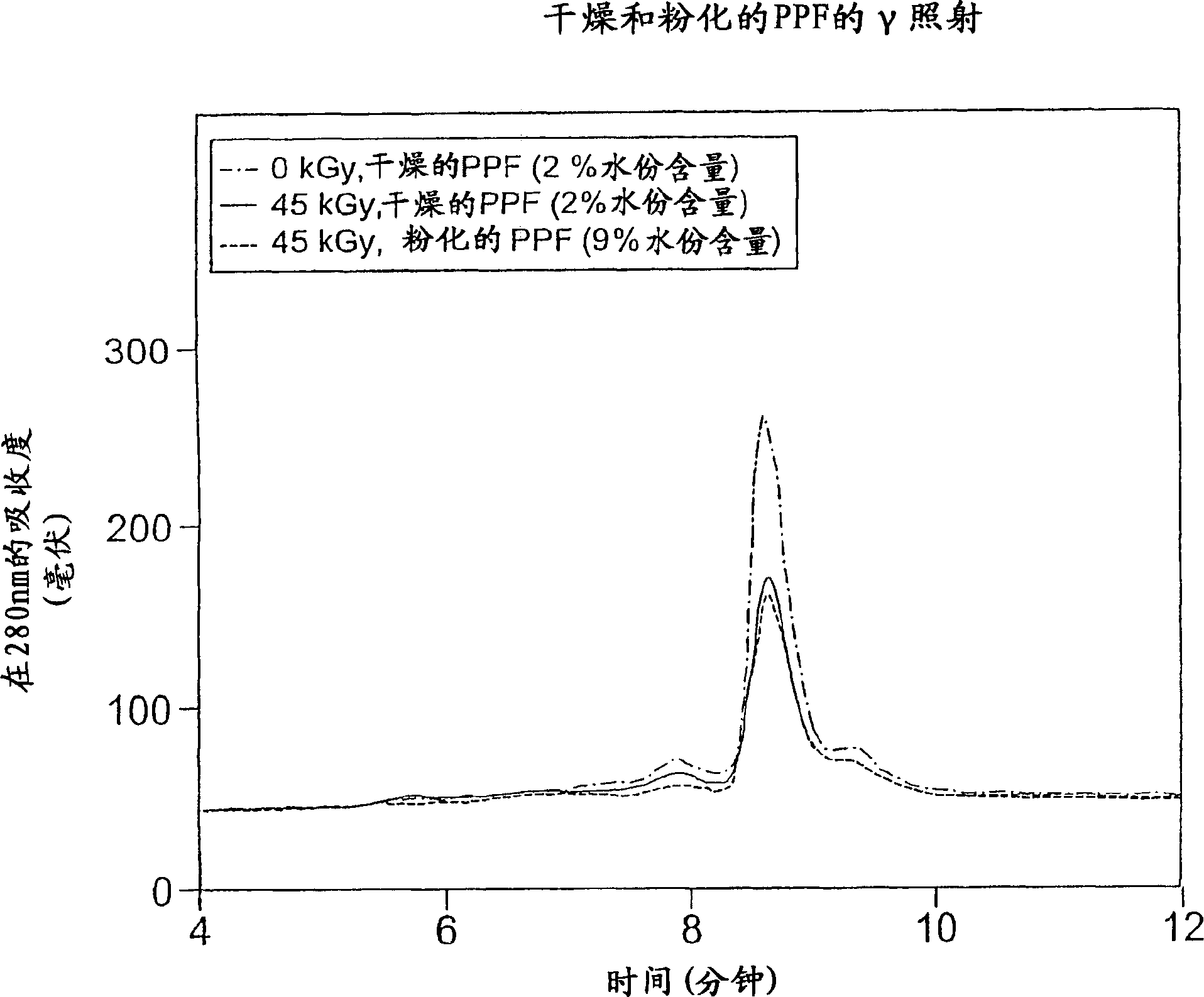
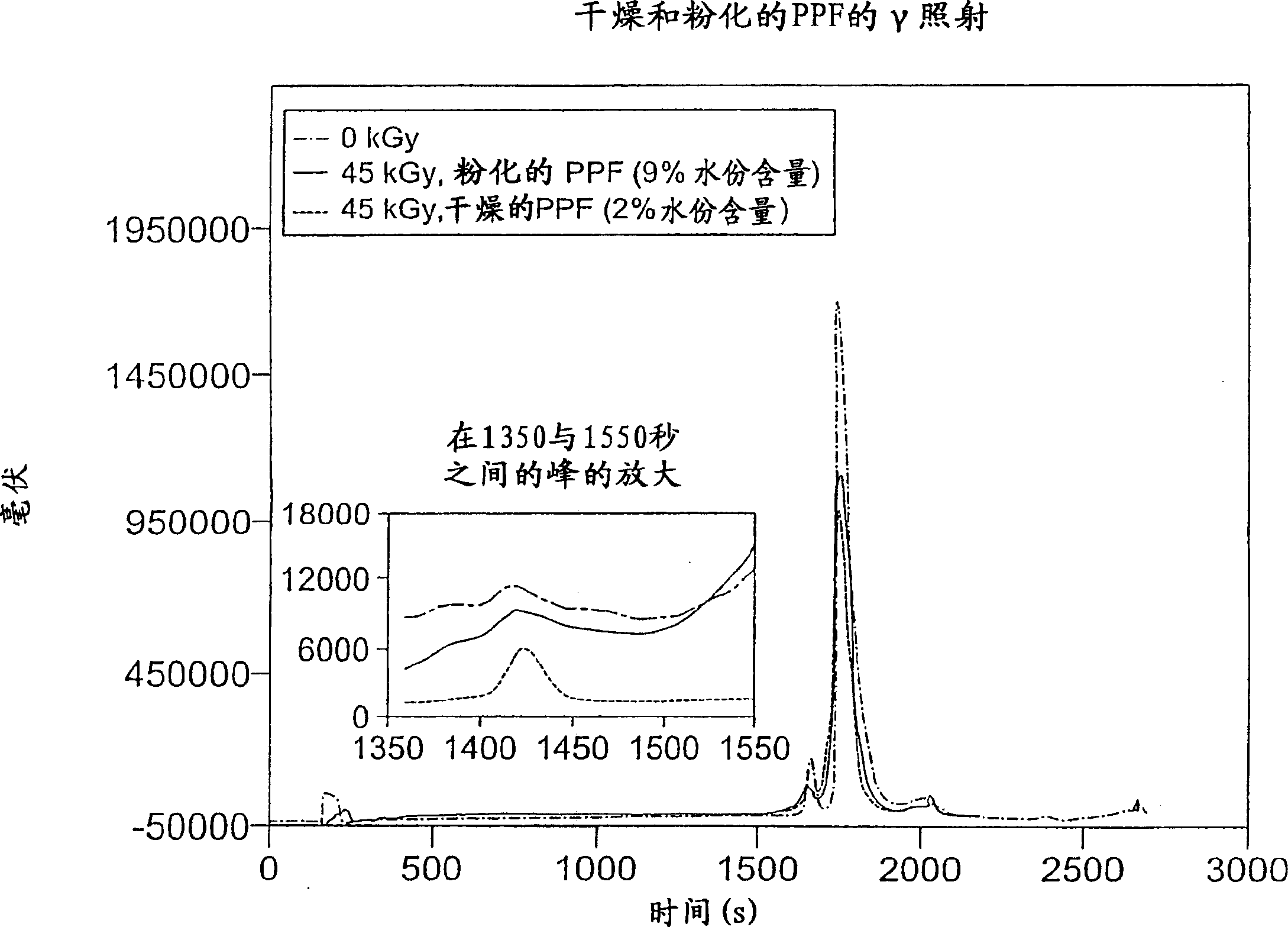
[0104] In this experiment, plasma protein fractions were irradiated (45 kGy, 1.9 kGy / hour, room temperature) at different levels of residual solvent content and in the presence or absence of volatile stabilizers.
[0105] method
[0106] In glass vials, samples of commercially available plasma protein fractions (2 mg / ml) were prepared with either 9% water with little ethanol and acetone, or 1% water substantially free of ethanol or acetone. Samples were irradiated with gamma rays (45 kGy total dose, 1.9 kGy / hour and room temperature) prior to structural integrity analysis. Structural integrity was determined by SDS-PAGE, HPLSEC and reverse phase HPLC.
[0107] For SDS-PAGE, prepare three 12.5% gels as follows: 4.2ml acrylamide; 2.5ml 4X-Tris (pH8.8); 3.3ml water; 100μl 10% APS solution; and 10μl TEMED In an electrophoresis unit with 1× Running Buffer (15.1 g Tris matrix; 72.0 g glycine; 5.0 g SDS in 1 liter of water, diluted 5-fold). Irradiated and control samples (1 mg / m...
Embodiment 2
[0114] Human albumin (25%) was infected with 10% brain homogenate from hamsters with altered scrapie (strain 263K) at a ratio of 1:100. The samples were mixed by vortexing, and four 6-ml aliquots of scrapie-infected albumin were dispensed into 10-ml serum vials. One vial was stored at -80°C as a frozen control. Place the three vials into commercially available radiation equipment. One vial (OkGy control) was refrigerated to prevent bacterial growth. The remaining vials were irradiated at room temperature (20-25° C.) at a rate of 0.4 kGy / hour to a total dose of 26 or 50 kGy. The radiation dose was determined with a dosimeter attached to each vial and an external dosimeter placed close to the vial. Scrapie infectivity of irradiated samples and 0 kGy control samples was determined.
[0115] Infectivity was determined by inoculating 0.05 ml samples into the brains of 12 hamsters, which were then fed for 6 months and observed. Three clinical endpoints were evaluated: shaking, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com