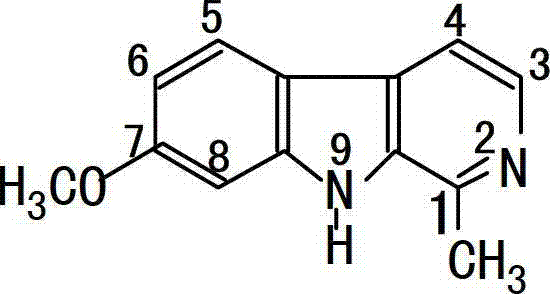
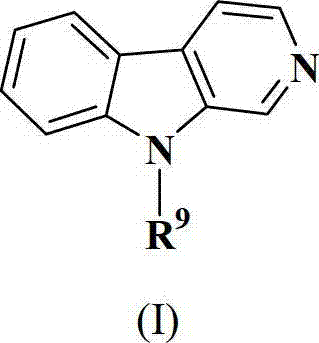
Application of Harmine derivative to preparation of antibacterial medicine
A technology of medicine and pharmacy, applied in the field of medicine, can solve the problems such as no report on the antibacterial activity of dehydrocamaprine derivatives and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A-1
[0999] General synthetic process of 2-substituted β-carboline alkaloid derivatives (201-206):
[1000] β-carboline (2mmol), add an appropriate amount of ethyl acetate to dissolve it completely, add the corresponding bromine / iodoalkane (10-20mmol), heat and reflux for 8 hours, cool to room temperature, filter the precipitated solid, wash with ethyl acetate , then dissolve the solid in absolute ethanol, heat to reflux until clarification, filter while it is hot, and place it in the refrigerator for recrystallization to obtain white or light yellow crystals.
[1001] 2-Benzyl-1-methyl-β-carboline bromide (201): starting from 1-methyl-β-carboline and benzyl bromide, a light yellow crystal (0.46g, 65%) was obtained, mp >270℃; FAB-MS m / e 273; IR(KBr) 3420,1750-3250,1634,1575,1525,1500,1452,1331,1299,1258,1229,1146,740; 1H-NMR(500MHz, DMSO-d6)δ12.94(1H,s,NH);8.80-8.82(1H,d,J=7.0Hz,H-3);8.73-8.75(1H,d,J=6.5Hz,H-4) ;8.48-8.50(1H,d,J=7.5Hz,H-5);7.79-7.80(2H,m,H-8,H-7);7.25-7.47(6H,m,H...
Embodiment A-2
[1009]
[1010] A general synthetic process for the 7-position alkylation reaction (208-215):
[1011] Mix 9-ethyl-1-methyl-β-carbolin-7-ol (2.0mmol), DMF (30ml), 60% sodium hydride (0.2g, 5mmol), stir at room temperature or under ice bath for 5min, then Add the corresponding halogenated alkanes (3-5mmol), stir and react for 0.5-2 hours, TLC tracking detection (developer: acetone / petroleum ether = 1:1), after the reaction is complete, pour the reaction mixture into water, add 10M sodium hydroxide , stirred at room temperature overnight, filtered, washed with a large amount of water, dissolved the solid in absolute ethanol, adjusted the pH to 3-4 with concentrated hydrochloric acid, concentrated under reduced pressure, added water to the absolute ethanol several times, and recrystallized from acetone or acetone / ether to obtain White or light yellow solid. Dissolve the solid in a mixed solution of ethyl acetate / water, basify with saturated sodium bicarbonate solution, extract ...
Embodiment A-3
[1023]
[1024] General synthetic procedure for 7-alkoxy-9-n-butyl-1-methyl-β-carboline derivatives (216-223):
[1025] Mix 9-n-butyl-1-methyl-β-carboline-7 alcohol (2.0mmol), DMF (30ml), 60% sodium hydride (0.2g, 5mmol), stir at room temperature or under ice bath for 5min, then Add the corresponding halogenated alkanes (3.5-5mmol), stir and react for 1-2 hours, TLC tracking detection (developer: acetone / petroleum ether = 1:1), after the reaction is complete, pour the reaction mixture into water, add 10M sodium hydroxide , stirred at room temperature overnight, filtered, washed with a large amount of water, dissolved the solid in absolute ethanol, adjusted the pH to 3-4 with concentrated hydrochloric acid, concentrated under reduced pressure, added water to the absolute ethanol several times, and recrystallized from acetone or acetone / ether to obtain White or light yellow solid. Dissolve the solid in a mixed solution of ethyl acetate / water, basify with sodium bicarbonate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com