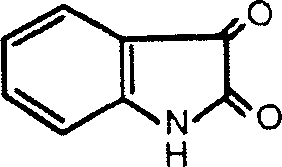
Application of indole-2,3-diketone in preparing medication for antivirus or immunopotenfiator
A technology of antiviral drugs and immune enhancers, which can be used in antiviral agents, pill delivery, pharmaceutical formulations, etc., and can solve problems such as normal cell damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Preparation of tablets
[0084] Mix ultrapure water and absolute ethanol at a ratio of 1:5, add synthetic indole-2,3-dione, slowly heat to dissolve at 60°C in a magnetic stirrer, and then gradually cool to form Needle-shaped orange-red crystals, repeat the above steps, repeat the crystallization 3 times to reduce heavy metal ions. Crush the indole-2,3-dione crystals into powder that has passed through an 80-mesh sieve, weigh out 50mg of indole-2,3-dione, 125mg of lactose, and 320mg of starch, mix well, granulate and dry by wet method, After sizing, it is mixed with 5 mg of magnesium stearate and compressed into tablets with a weight of 500 mg per tablet.
Embodiment 2
[0085] Example 2: Preparation of capsules
[0086] Mix ultrapure water and absolute ethanol at a ratio of 1:5, add synthetic indole-2,3-dione, slowly heat to dissolve at 70°C in a magnetic stirrer, and then gradually cool to a needle Shape orange-red crystals, repeat the above steps, repeat the crystallization 3 times to reduce heavy metal ions. Crush the indole-2,3-dione crystals into powder that has passed an 80-mesh sieve, weigh out 50mg of indole-2,3-dione, 445mg of starch, and 5mg of magnesium stearate, mix well, and fill the capsules. Prepare capsules each containing 500mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com